基于 ReaxFF MD 模拟和实验对 Ca2SiO4 直接碳化过程的深入研究
IF 10.9
1区 工程技术
Q1 CONSTRUCTION & BUILDING TECHNOLOGY
引用次数: 0
摘要
Ca2SiO4 是钢渣中主要的碳化反应矿物,具有显著的固碳潜力,但其微观反应过程仍不清楚。本研究利用 ReaxFF MD 模拟研究了 Ca2SiO4 的碳化行为。结果表明,随着二氧化碳浓度的增加,Ca2SiO4 的捕获率降低,生成的 CaCO3 分子结构因氧源而异。在室温下,Ca₂SiO₄ 的碳化率随着时间的推移逐渐降低,直至达到平衡。提高温度可以重新激活碳化,但碳化速率仍会下降,直至再次达到平衡。更高的温度可加速中间体 C2O52- 的形成和内部 CO32- 的扩散,从而促进碳化和增加 CO2 吸附。本研究从原子水平研究了 Ca2SiO4 的碳化过程,旨在将微观分子过程与宏观实验现象联系起来,从而为提高钢渣的碳化效率提供理论依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
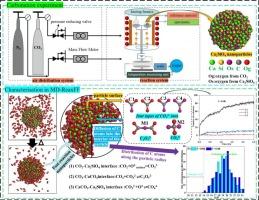
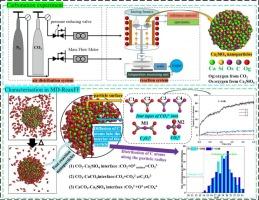
Insight into the direct carbonation process of Ca2SiO4 based on ReaxFF MD simulation and experiments
Ca2SiO4 is the primary carbonation-reactive mineral in steel slag, and demonstrates significant carbon sequestration potential, yet its microscopic reaction processes remain unclear. This study investigated the carbonation behavior of Ca2SiO4 using ReaxFF MD simulations. The results indicated that as CO2 concentration increased, the capture rate of Ca2SiO4 decreased, and the molecular structure of the resulting CaCO3 varied in oxygen origin. At room temperature, the carbonation rate of Ca₂SiO₄ gradually decreased over time until it reached equilibrium. Increasing the temperature could reactivate the carbonation, but the rate would still decline until it reached equilibrium again. Higher temperatures could accelerate the formation of the intermediate C2O52− and internal CO32− diffusion, thereby boosting the carbonation and increasing CO2 adsorption. This study investigated the carbonation of Ca2SiO4 at the atomic level, aiming to link microscopic molecular processes with macroscopic experimental phenomena, thereby providing a theoretical foundation for enhancing the carbonation efficiency of steel slag.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cement and Concrete Research
工程技术-材料科学:综合
CiteScore
20.90
自引率
12.30%
发文量
318
审稿时长
53 days
期刊介绍:
Cement and Concrete Research is dedicated to publishing top-notch research on the materials science and engineering of cement, cement composites, mortars, concrete, and related materials incorporating cement or other mineral binders. The journal prioritizes reporting significant findings in research on the properties and performance of cementitious materials. It also covers novel experimental techniques, the latest analytical and modeling methods, examination and diagnosis of actual cement and concrete structures, and the exploration of potential improvements in materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


