新型辅酶 Q6 基因变异会增加肺炎球菌疾病的易感性
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
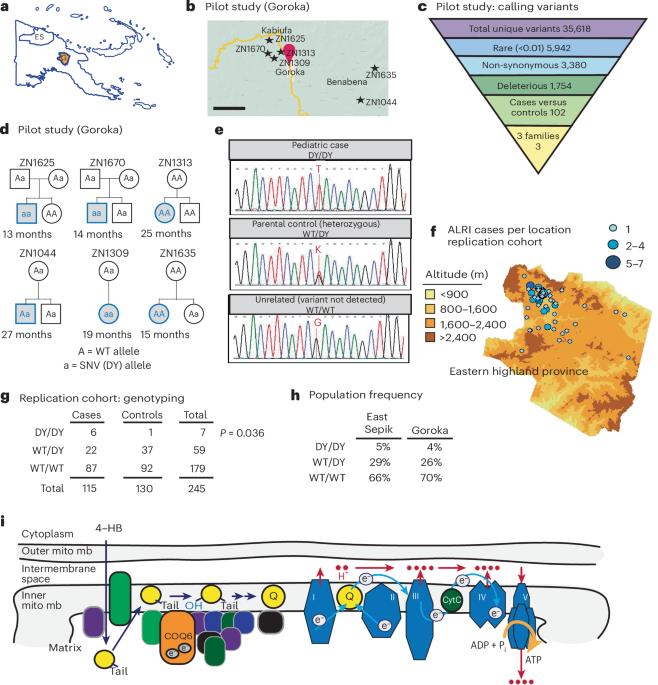
急性下呼吸道感染(ALRI)仍然是全球儿童死亡的主要原因,因此必须在预防和治疗方面进行创新。巴布亚新几内亚(PNG)儿童因肺炎链球菌引起的急性下呼吸道感染而发病率极高。由于进化分化,巴布亚新几内亚的人类群体呈现出深刻的遗传变异和多样性。为了满足巴布亚新几内亚儿童尚未得到满足的健康需求,我们测试了基因变异是否会增加 ALRI 的发病率。对试点儿童队列进行的全外显子组测序发现,在患有 ALRI 的病例中,辅酶 Q6(COQ6)中存在一种新型单核苷酸变异(SNV)。COQ6 是一种线粒体酶,对电子传递链中电子受体泛醌的生物合成至关重要。在一个独立的 ALRI 队列中,SNV 同源基因与 ALRI 有着明显的关联(P = 0.036)。同源小鼠变异体 Coq6 的同源小鼠在肺炎球菌肺部感染后死亡率增加,证实了这一因果关系。骨髓嵌合小鼠进一步表明,变异型 Coq6 在受体(即非造血组织)中的表达会增加死亡率。变体 Coq6 可维持泛醌的生物合成,同时在肺炎球菌挑战后加速新陈代谢的重塑。COQ6变体的鉴定为PNG肺炎易感性的增加提供了遗传基础,并确定了COQ6酶在调节炎症介导的新陈代谢重塑中以前未被认识到的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Novel coenzyme Q6 genetic variant increases susceptibility to pneumococcal disease
Acute lower respiratory tract infection (ALRI) remains a major worldwide cause of childhood mortality, compelling innovation in prevention and treatment. Children in Papua New Guinea (PNG) experience profound morbidity from ALRI caused by Streptococcus pneumoniae. As a result of evolutionary divergence, the human PNG population exhibits profound genetic variation and diversity. To address unmet health needs of children in PNG, we tested whether genetic variants increased ALRI morbidity. Whole-exome sequencing of a pilot child cohort identified homozygosity for a novel single-nucleotide variant (SNV) in coenzyme Q6 (COQ6) in cases with ALRI. COQ6 encodes a mitochondrial enzyme essential for biosynthesis of ubiquinone, an electron acceptor in the electron transport chain. A significant association of SNV homozygosity with ALRI was replicated in an independent ALRI cohort (P = 0.036). Mice homozygous for homologous mouse variant Coq6 exhibited increased mortality after pneumococcal lung infection, confirming causality. Bone marrow chimeric mice further revealed that expression of variant Coq6 in recipient (that is, nonhematopoietic) tissues conferred increased mortality. Variant Coq6 maintained ubiquinone biosynthesis, while accelerating metabolic remodeling after pneumococcal challenge. Identification of this COQ6 variant provides a genetic basis for increased pneumonia susceptibility in PNG and establishes a previously unrecognized role for the enzyme COQ6 in regulating inflammatory-mediated metabolic remodeling. Morley, Pomat and colleagues identify a homozygous mutation in COQ6 in an indigenous population of Papua New Guinea. This allele predisposes children to increased risk of acute lower respiratory infections in response to Streptococcus pneumoniae. This sensitivity is recapitulated in Coq6DY mice in the nonhematopoietic compartment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


