在嵌合型阿尔茨海默病模型中,消耗小胶质细胞可减少人类神经元与 APOE4 相关的病变
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
尽管有强有力的证据支持脂蛋白E4(APOE4)和小胶质细胞在阿尔茨海默病(AD)发病机制中的重要作用,但小胶质细胞对神经元APOE4相关AD发病机制的影响仍然难以捉摸。为了研究这种影响,我们在小鼠海马与诱导多能干细胞(iPSC)衍生的人类神经元的嵌合模型中利用了小胶质细胞耗竭。具体来说,我们将同基因 APOE4、同基因 APOE3 和 APOE 基因敲除(APOE-KO)iPSC 衍生的人类神经元移植到人类 APOE3 或 APOE4 基因敲除小鼠的海马中,然后在一半的嵌合小鼠中消耗小胶质细胞。我们发现,神经元 APOE 和小胶质细胞的存在对 Aβ 和 tau 病理学的形成都很重要,而这种形成是以 APOE 同工酶依赖性(APOE4 > APOE3)的方式进行的。单细胞 RNA 测序分析发现,在人类 APOE4 神经元移植的嵌合小鼠中,有两种促炎性小胶质细胞亚型的 MHC-II 基因表达增高。这些发现凸显了神经元 APOE(尤其是 APOE4)和小胶质细胞在注意力缺失症发病机制中的协同作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
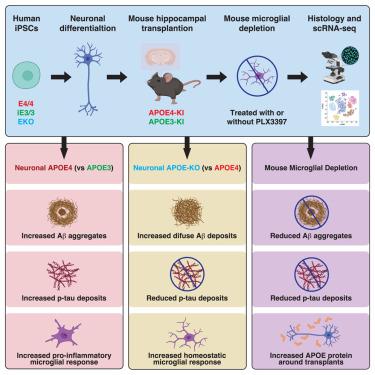
Microglia depletion reduces human neuronal APOE4-related pathologies in a chimeric Alzheimer’s disease model
Despite strong evidence supporting the important roles of both apolipoprotein E4 (APOE4) and microglia in Alzheimer’s disease (AD) pathogenesis, the effects of microglia on neuronal APOE4-related AD pathogenesis remain elusive. To examine such effects, we utilized microglial depletion in a chimeric model with induced pluripotent stem cell (iPSC)-derived human neurons in mouse hippocampus. Specifically, we transplanted homozygous APOE4, isogenic APOE3, and APOE-knockout (APOE-KO) iPSC-derived human neurons into the hippocampus of human APOE3 or APOE4 knockin mice and then depleted microglia in half of the chimeric mice. We found that both neuronal APOE and microglial presence were important for the formation of Aβ and tau pathologies in an APOE isoform-dependent manner (APOE4 > APOE3). Single-cell RNA sequencing analysis identified two pro-inflammatory microglial subtypes with elevated MHC-II gene expression enriched in chimeric mice with human APOE4 neuron transplants. These findings highlight the concerted roles of neuronal APOE, especially APOE4, and microglia in AD pathogenesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


