光营养细菌 Chloroflexus aurantiacus 的替代复合体 III 的晶体结构
IF 4.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
交替复合体 III(ACIII)是一种多亚基醌:电子受体氧化还原酶,在细菌呼吸和光合电子传递链中将醌氧化与跨膜质子转运结合在一起。目前已知四种 ACIII 冷冻电镜(cryo-EM)结构。然而,低温电子显微镜与 X 射线晶体学结构测定对 ACIII 结构的影响尚不清楚。在此,我们报告了来自 Chloroflexus aurantiacus 的光合作用 ACIII(CaACIIIp)的 3.25 Å 晶体结构,揭示了八个亚基(ActA-G 和 I),其中有四个铁硫簇和六个 c 型血红素、一个甲萘醌结合位点和两个质子转运通道。与之前报道的低温电子显微镜结构比较发现,ActB、ActD、ActG 的溶剂暴露区和亚基 I 的跨膜(TM)螺旋有轻微的局部构象变化。不过,包含甲萘醌结合袋、氧化还原中心和质子转运通道的核心功能模块保持不变,从而保持了酶的活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
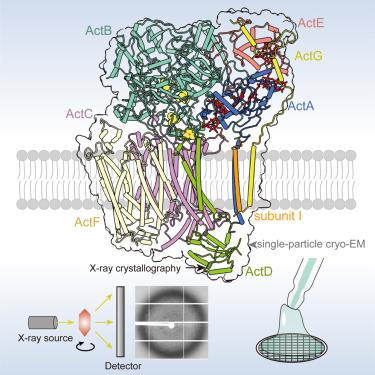
Crystal structure of the alternative complex III from the phototrophic bacterium Chloroflexus aurantiacus
Alternative complex III (ACIII) is a multi-subunit quinol:electron acceptor oxidoreductase that couples quinol oxidation with transmembrane proton translocation in bacterial respiratory and photosynthetic electron transport chains. Four ACIII cryoelectron microscopy (cryo-EM) structures are known. However, the effects of cryo-EM versus X-ray crystallography structure determination on ACIII structure are unclear. Here, we report a 3.25 Å crystal structure of photosynthetic ACIII from Chloroflexus aurantiacus (CaACIIIp), revealing eight subunits (ActA–G and I) with four iron-sulfur clusters and six c-type hemes, a menaquinol-binding site, and two proton translocation passages. Structural comparisons with the previously reported cryo-EM structures reveal slight local conformational changes in the solvent-exposed regions of ActB, ActD, ActG, and the transmembrane (TM) helix of subunit I. The regions conferring structural flexibility possess low sequence conservation across species. However, the core functional modules containing the menaquinol-binding pocket, redox centers, and proton translocation passages remain unchanged, preserving the enzyme’s activity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


