人类 TRPV6 祖先和衍生单倍型的结构功能分析
IF 4.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
TRPV6 是一种 Ca2+ 选择性通道,介导肠道中的钙吸收,并导致人类癌症的发生和发展。TRPV6 由祖先型和衍生单倍型代表,这两种单倍型因三个非同义多态性而不同,分别位于 N 端 ankyrin 重复结构域(C157R)、S1-S2 细胞外环(M378V)和 C 端(M681T)。这些祖先单倍型和衍生单倍型被认为是导致非洲裔癌症患者不同预后的基因组因素。我们利用冷冻电子显微镜(cryo-EM)解决了开放状态和与钙调蛋白(CaM)结合的失活状态下祖先和衍生 TRPV6 的结构。这两种状态都没有显示出非同义多态性导致的实质性结构差异。通过电生理记录和 Ca2+ 摄取测量评估的功能特性,以及通过分子建模评估的水和离子渗透性在单倍型之间似乎也很相似。因此,祖先和衍生的 TRPV6 具有相似的结构和功能,这意味着癌症易感性的差异是由其他因素造成的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
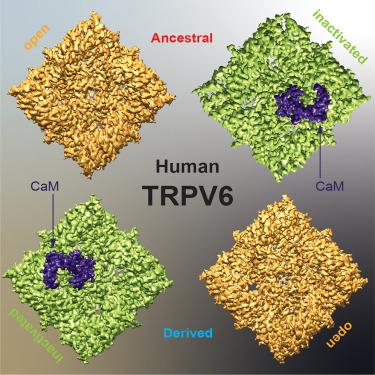
Structure-function analyses of human TRPV6 ancestral and derived haplotypes
TRPV6 is a Ca2+ selective channel that mediates calcium uptake in the gut and contributes to the development and progression of human cancers. TRPV6 is represented by the ancestral and derived haplotypes that differ by three non-synonymous polymorphisms, located in the N-terminal ankyrin repeat domain (C157R), S1–S2 extracellular loop (M378V), and C-terminus (M681T). The ancestral and derived haplotypes were proposed to serve as genomic factors causing a different outcome for cancer patients of African ancestry. We solved cryoelectron microscopy (cryo-EM) structures of ancestral and derived TRPV6 in the open and calmodulin (CaM)-bound inactivated states. Neither state shows substantial structural differences caused by the non-synonymous polymorphisms. Functional properties assessed by electrophysiological recordings and Ca2+ uptake measurements, and water and ion permeation evaluated by molecular modeling also appear similar between the haplotypes. Therefore, ancestral and derived TRPV6 have similar structure and function, implying that other factors are responsible for the differences in susceptibility to cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


