未活化烷烃的烯化反应:通过双 Co-TBADT 催化合成 Z-烯烃
IF 4.3
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
烯烃是有机合成的基本构件。通过 C-H 活化末端炔烃进行氢烷基化可以说是合成烯烃的最简单、最经济的方法,但它们仍然受限于使用 C(sp2)-H 或活化的 C(sp3)-H 键。虽然螯合基团促进了 C(sp3)-H 键的烯化,但却产生了 E 烯。在没有螯合基团的情况下,通过金属酸酐或自由基途径直接对未活化的 C(sp3)-H 键进行烯化的方法仍然未知。尽管对镍-HAT 催化的全面评估结果并不理想,但我们的研究展示了钴-HAT 催化的潜力,即通过炔烃 SOMOphiles 与原位生成的开壳烷基自由基反应,以优异的区域和非对映选择性获得所需的 Z 烯。在初步研究的基础上,设计出了一种机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
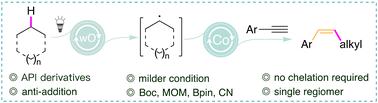
Alkenylation of unactivated alkanes: synthesis of Z-alkenes via dual Co-TBADT catalysis†
Hydroalkylation of terminal alkynes via C–H activation is the most atom-economical and straightforward method for synthesizing alkenes. They remain confined to using C(sp2)–H or activated C(sp3)–H bonds. A chelating group enabled the alkenylation of C(sp3)–H bonds, resulting in E alkenes. Protocols by which alkenylation of unactivated C(sp3)–H bonds occurs without a chelating group via metal-hydride or radical pathways remain unknown. Our cobalt-HAT catalysis achieves the desired Z alkene with excellent regio- and diastereoselectivity via C–H activation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


