通过分子动力学模拟和实验研究实现铜与莫来石和沸石的离子交换
IF 4.8
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
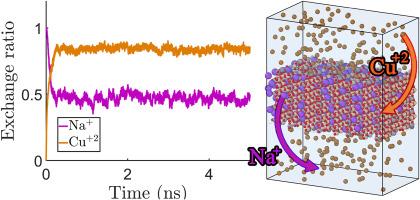
由于铜交换沸石可用作选择性氧化还原催化剂和传感器,因此对其进行了多项研究。然而,不同类型的沸石交换铜离子的能力仍存在争议。本研究采用全原子分子动力学(MD)模拟、能量色散 X 射线光谱(EDS)和 X 射线荧光(XRF)方法,通过水溶液法评估了莫来石和沸石中 Cu(II)的交换。测量了两种沸石中铜离子的几个参数,如离子交换率、均方位移(MSD)、扩散系数和径向分布函数(RDF)。在进料溶液中铜离子浓度不同的情况下,对每种沸石系统的这些参数进行了计算。铜交换比和 RDF 分析表明,铜离子在莫来石框架中的交换比更高。势能分析表明,莫来石和鳞片沸石的主要吸附位点位于沸石的最大空腔中。莫来石位点的吸附能(1.52 eV)大于铮沸石(1.28 eV)。铜离子与莫来石位点之间更强的吸引力与该沸石中较低的离子扩散系数相一致。使用 EDS 分析法检测了沸石的离子交换能力。根据 EDS 分析结果,莫代沸石的铜离子交换率高于沸石,这与计算结果一致。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ion-exchange of copper into mordenite and clinoptilolite zeolites by molecular dynamics simulations and experimental investigations
Copper-exchanged zeolites have been subjected to several investigations because of their application as selective redox-active catalysts, and sensors. However, the ability of different types of zeolites to exchange Cu ions remains a matter of debate. All-atom molecular dynamics (MD) simulations, energy dispersive X-ray spectroscopy (EDS), and X-ray Fluorescence (XRF) methods have been used to evaluate the exchange of Cu(II) in mordenite and clinoptilolite zeolites using an aqueous method in the current study. Several parameters of copper ions have been measured for both types of zeolites, such as ion exchange ratio, mean square displacement (MSD), diffusion coefficient, and radial distribution function (RDF). These parameters were calculated for each zeolite system at different Cu ion concentrations in the feed solution. Copper exchange ratio and RDF analyses revealed a higher exchange ratio of copper ions in the mordenite framework. Analysis of the potential energy indicates the major adsorption sites for mordenite and clinoptilolite, which are located in the largest cavities of the zeolites. The adsorption energy of the mordenite sites (1.52 eV) was larger than that of clinoptilolite (1.28 eV). The stronger attraction between the copper ions and mordenite sites is consistent with the lower diffusion coefficients of the ions in this zeolite. The ion-exchange abilities of the zeolites were examined using EDS analysis. According to the EDS results, the mordenite zeolite exchanged more copper ions than the clinoptilolite, which is in agreement with the computational results.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


