第一种碱金属/碱土金属草磷酸盐 Na4Mg3(HPO4)4(C2O4)-2H2O 的合成、结构和特性分析
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
采用常规溶热法成功合成了首个具有单斜 P21/n 空间群的碱金属/碱土金属草磷酸盐 Na4Mg3(HPO4)4(C2O4)-2H2O。Na4Mg3(HPO4)4(C2O4)-2H2O晶体呈现出典型的三维结构,由Na和Mg原子连接的孤立的[C2O4]和[HPO4]阴离子基团构成。HPO42- 基团通过氢键连接,形成独特的一维之字带 ∞[HPO4]2-,在 bc 平面内呈现稳定的 "莫蒂斯-噻农 "结构构型。傅立叶变换红外光谱和拉曼光谱证实了 Na4Mg3(HPO4)4(C2O4)-2H2O 的晶体结构。紫外可见近红外(UV-Vis-NIR)漫反射和能带结构计算表明,Na4Mg3(HPO4)4(C2O4)-2H2O 是一种间接带隙半导体,带隙为 4.42 eV。此外,Na4Mg3(HPO4)4(C2O4)-2H2O 在 550 纳米波长处显示出 0.046 的中等双折射。本文章由计算机程序翻译,如有差异,请以英文原文为准。
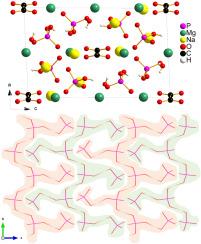
Synthesis, structure and characterizations of the first alkali-metal/alkaline-earth-metal oxalatophosphate Na4Mg3(HPO4)4(C2O4)·2H2O
The first alkali-metal/alkaline-earth-metal oxalatophosphate Na4Mg3(HPO4)4(C2O4)·2H2O with monoclinic space group was successfully synthesized by a conventional solvothermal method. Na4Mg3(HPO4)4(C2O4)·2H2O crystal exhibits a typical three-dimensional structure built by isolated [C2O4] and [HPO4] anionic groups connected by Na and Mg atoms. The HPO42− groups are linked by hydrogen bonds to form an unique one-dimensional zigzag band ∞[HPO4]2-, which exhibits a stable “Mortise-Tenon” structure configuration within the bc plane. Fourier transform infrared (FTIR) spectrum and Raman spectrum confirm the crystal structure of Na4Mg3(HPO4)4(C2O4)·2H2O. UV–visible near infrared (UV–Vis–NIR) diffuse reflectance and band structure calculation indicate that Na4Mg3(HPO4)4(C2O4)·2H2O is an indirect bandgap semiconductor with a bandgap of 4.42 eV. In addition, Na4Mg3(HPO4)4(C2O4)·2H2O shows a moderate birefringence of 0.046 at 550 nm.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


