使用二羧酸添加剂改善水性镁-空气电池的放电性能
IF 5.3
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
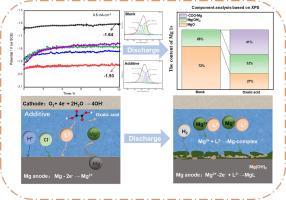
镁-空气水电池在储能系统中具有广阔的应用前景。为了改善镁阳极的放电性能,草酸、苹果酸、琥珀酸和己二酸等四种二羧酸被用作水基镁-空气电池的电解质添加剂。通过氢演化、电化学实验(OCP、半/全电池测试、EIS)、扫描电镜、XRD、XPS 和 DFT 计算,系统研究了所选四种添加剂的影响及其反应机理。实验结果表明,二羧酸电解质添加剂通过增加氢气进化量和放电电位负移,明显提高了镁阳极的活性。表面形貌和化学成分分析表明,所选的四种添加剂都能与 Mg2+ 形成可溶性络合物。草酸是四种二羧酸中最好的电解质添加剂。添加剂的螯合能力和电解液的 pH 值都会影响镁阳极的放电性能。二羧酸添加剂改善镁阳极放电性能的机理得到了 DFT 计算的进一步证实。正是添加剂与镁阳极之间形成的可溶性 Mg2+ 复合物(MgL,L:二羧酸盐)抑制了腐蚀产物的形成,促进了镁阳极的溶解,从而提高了镁阳极的放电性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Improving the discharge performance of aqueous Mg-air battery using dicarboxylic acid additives
The aqueous Mg-air battery has promising applications in energy storage system. To improve the discharge performance of the Mg anode, four dicarboxylic acids including oxalic acid, malic acid, succinic acid, adipic acid are used as electrolyte additive for the aqueous Mg-air battery. The influence of the four selected additives and their reaction mechanism are systematically investigated by hydrogen evolution, electrochemical experiments (OCP, half/full-cell test, EIS), SEM, XRD, XPS and DFT calculations. The experiment results demonstrate that the dicarboxylic acid electrolyte additive can active the Mg anode evidently by increasing the hydrogen evolution volume and negative shift of discharge potentials. The surface morphology and the chemical composition analyses demonstrate that the four selected additives can form soluble complexes with Mg2+. The oxalic acid is the best electrolyte additive among the four dicarboxylic acids. The chelating ability of the additives and the pH of the electrolyte both affect the discharge performance of the Mg anode. The mechanism by which the dicarboxylic acids additives improve the discharge performance of the Mg anode is further proved by DFT calculations. It is the soluble Mg2+ complex (MgL, L: dicarboxylate) formed between the additives and the Mg anode that inhibit the formation of the corrosion products and promote the dissolution of the Mg anode, thus enhance the discharge performance of the Mg anode.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Research Bulletin
工程技术-材料科学:综合
CiteScore
9.80
自引率
5.60%
发文量
372
审稿时长
42 days
期刊介绍:
Materials Research Bulletin is an international journal reporting high-impact research on processing-structure-property relationships in functional materials and nanomaterials with interesting electronic, magnetic, optical, thermal, mechanical or catalytic properties. Papers purely on thermodynamics or theoretical calculations (e.g., density functional theory) do not fall within the scope of the journal unless they also demonstrate a clear link to physical properties. Topics covered include functional materials (e.g., dielectrics, pyroelectrics, piezoelectrics, ferroelectrics, relaxors, thermoelectrics, etc.); electrochemistry and solid-state ionics (e.g., photovoltaics, batteries, sensors, and fuel cells); nanomaterials, graphene, and nanocomposites; luminescence and photocatalysis; crystal-structure and defect-structure analysis; novel electronics; non-crystalline solids; flexible electronics; protein-material interactions; and polymeric ion-exchange membranes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


