通过聚多巴胺衍生的碳涂层构建坚固的纳米铜结构,用于氧还原反应
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
本研究探讨了铜单原子/簇嵌入氮掺杂碳与 Cu/Cu2O 纳米颗粒(Cu-X-NC)形成的铜氮碳复合材料的合成与电催化性能,用于氧还原反应(ORR)。催化剂以多多巴胺为碳源和氮源,通过溶解热碳化(STC)法合成,然后进行热解和酸洗。研究了溶热碳化温度(120、150 和 180 °C)对催化剂结构和 ORR 活性的影响。理化表征结果表明,较高的 STC 温度会减小铜晶体的尺寸,略微增加铜(I)氧化物的形成,并在 150 ℃ 时形成分散良好的铜单原子/簇。这种最佳分散性增强了铜单质与反应物之间的相互作用,从而加快了 ORR 动力学,电化学阻抗谱测量中较低的电荷转移电阻值就证明了这一点。此外,在这一温度下,微孔和中孔结构之间的平衡促进了有效的质量传输,这对实现更高的 ORR 活性至关重要。此外,加速稳定性测试表明,Cu-150-NC 具有出色的耐久性,在 10,000 次循环后,起始电位的损失可以忽略不计。溶热工艺显著增加了电化学活性表面积(ECSA),Cu-150-NC 显示出最高的比活度和每克铜的质量活度,表明其性能优越。总之,这些发现强调了合成优化的重要性,并为设计燃料电池应用的环保型高性能铜催化剂提供了宝贵的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
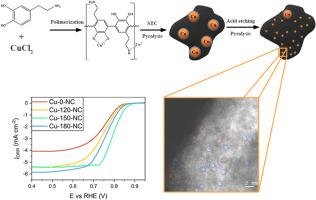
Building robust copper nanostructures via carbon coating derived from polydopamine for oxygen reduction reaction
This study explores the synthesis and electrocatalytic performance of copper-nitrogen-carbon composites formed by Cu single atoms/clusters embedded in nitrogen-doped carbon with Cu/Cu2O nanoparticles (Cu-X-NC) for the oxygen reduction reaction (ORR). The catalysts were synthesized using polydopamine as a carbon and nitrogen source via the solvothermal carbonization (STC) method, followed by pyrolysis and acid washing. The effect of solvothermal carbonization temperature (120, 150, and 180 °C) on the structure and ORR activity was investigated. The physicochemical characterization showed that higher STC temperatures reduced the size of copper crystallites, slightly increased the formation of copper(I) oxide, and led to the creation of well-dispersed copper single atoms/clusters at 150 °C. This optimal dispersion enhances the interaction between the copper single atoms and the reactants, leading to faster ORR kinetics, as demonstrated by the lower charge transfer resistance values in electrochemical impedance spectroscopy measurements. Additionally, the balance between micropore and mesopore structures at this temperature facilitates efficient mass transport, which is critical for achieving higher ORR activity. Moreover, accelerated stability tests showed excellent durability for Cu-150-NC, with negligible loss in onset potential after 10,000 cycles. The solvothermal process significantly increased the electrochemically active surface area (ECSA), with Cu-150-NC displaying the highest specific activity and mass activity per gram of copper, indicating superior performance. Overall, these findings underscore the importance of synthesis optimization and provide valuable insights for designing eco-friendly and high-performance copper catalysts for fuel cell applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Nano
Multiple-
CiteScore
11.30
自引率
3.90%
发文量
130
审稿时长
31 days
期刊介绍:
Materials Today Nano is a multidisciplinary journal dedicated to nanoscience and nanotechnology. The journal aims to showcase the latest advances in nanoscience and provide a platform for discussing new concepts and applications. With rigorous peer review, rapid decisions, and high visibility, Materials Today Nano offers authors the opportunity to publish comprehensive articles, short communications, and reviews on a wide range of topics in nanoscience. The editors welcome comprehensive articles, short communications and reviews on topics including but not limited to:
Nanoscale synthesis and assembly
Nanoscale characterization
Nanoscale fabrication
Nanoelectronics and molecular electronics
Nanomedicine
Nanomechanics
Nanosensors
Nanophotonics
Nanocomposites
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


