基于 PEG 的 Elesclomol@Cu(Ⅱ)金属有机框架具有有效的纳米酶性能和杯突症诱导功效,可用于增强基于 PD-L1 的免疫疗法
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
纳米酶是一种很有前景的抗肿瘤治疗策略。然而,基于金属有机框架(MOF)的纳米酶在杯突变过程中的催化功能仍不清楚。本研究开发了一种装载了铜离子诱导剂伊来克洛莫尔并经聚乙二醇聚合物(PEG)表面修饰的基于Cu(Ⅱ)的MOF纳米复合材料(ES@Cu(Ⅱ)-MOF),用于有效诱导杯突酶变。ES@Cu(Ⅱ)-MOF的过氧化物酶(POD)样活性通过芬顿样反应产生大量羟自由基(-OH),其谷胱甘肽过氧化物酶(GSH-Px)样活性将Cu2+转化为毒性更强的Cu+离子,从而有效消耗内源性GSH。值得注意的是,Cu+和ES在肿瘤细胞中的快速积累会诱导脂酰化二氢脂酰胺S-乙酰转移酶(DLAT)的聚集和Fe-S簇蛋白的下调,最终导致杯突症。ES@Cu(Ⅱ)-MOF在体外对乳腺癌细胞具有超强的细胞毒性,在体内能显著抑制4T1乳腺肿瘤的生长。此外,ES@Cu(Ⅱ)-MOF 还能诱导免疫原性细胞死亡(ICD),从而增强抗肿瘤免疫反应。此外,将ES@Cu(Ⅱ)-MOF与抗程序性细胞死亡配体1(PD-L1)抗体结合使用,可将免疫抑制性肿瘤微环境转化为免疫原性微环境,从而有效抑制乳腺肿瘤的生长。总之,这项工作提供了一种利用纳米酶促进杯突酶作用治疗癌症的创新方法,有可能提高基于免疫检查点抑制剂的免疫疗法的有效性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
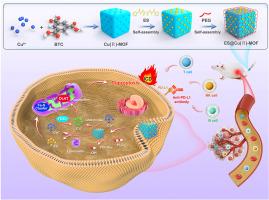
PEGylated Elesclomol@Cu(Ⅱ)-based Metal‒organic framework with effective nanozyme performance and cuproptosis induction efficacy for enhanced PD-L1-based immunotherapy
Nanozymes constitute a promising treatment strategy for antitumor therapy. However, the catalytic function of metal‒organic framework (MOF)-based nanozymes during cuproptosis remains unclear. In this study, a Cu(Ⅱ)-based MOF nanocomposite loaded with the copper ionophore elesclomol and surface modified with polyethylene glycol polymer (PEG) was developed (ES@Cu(Ⅱ)-MOF) for effective cuproptosis induction. The peroxidase (POD)-like activity of ES@Cu(Ⅱ)-MOF generated an abundance of hydroxyl radicals (•OH) via a Fenton-like reaction, and its glutathione peroxidase (GSH-Px)-like activity converted Cu2+ into more toxic Cu+ ions to efficiently consume endogenous GSH. Notably, the rapid accumulation of Cu+ and ES in tumor cells induced the aggregation of lipoylated dihydrolipoamide S-acetyltransferase (DLAT) and the downregulation of Fe‒S cluster proteins, ultimately leading to cuproptosis. ES@Cu(Ⅱ)-MOF exhibited extraordinary cytotoxicity against breast cancer cells in vitro and significantly suppressed 4T1 breast tumor growth in vivo. Moreover, ES@Cu(Ⅱ)-MOF induced immunogenic cell death (ICD) to increase the antitumor immune response. Furthermore, combining ES@Cu(Ⅱ)-MOF with an anti-programmed cell death-ligand 1 (PD-L1) antibody converted the immunosuppressive tumor microenvironment to an immunogenic microenvironment, thus effectively inhibiting breast tumor growth. Overall, this work provides an innovative approach utilizing nanozymes to facilitate cuproptosis for cancer treatment, which potentially enhances the effectiveness of immune checkpoint inhibitor-based immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


