使用纳米棒状过磷酸盐铯钛溴化物 (CsTiBr3) 通过集体降解净化纺织废水
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
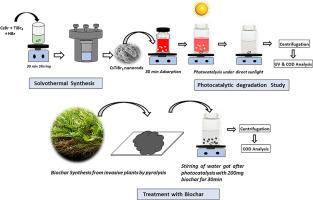
从纺织品染色单位排出的废水中含有多种染料混合物。在这里,我们要解决的难题是使用单一催化剂在阳光直射下集体光催化降解从当地染厂收集的六种染料混合物和两种纺织废水样品。通过溶热法制备的 CsTiBr3 纳米棒无铅过氧化物能有效地对含有刚果红、水晶紫、孔雀石绿、亚甲基蓝、罗丹明和甲基橙等有毒染料的染料混合物进行集体光催化降解。六种染料混合物在阳光直射三小时内变成无色。CsTiBr3 包晶纳米棒的集体光催化降解能力被成功地用于降解纺织污水。化学需氧量(COD)分析保证了染料降解为无毒成分。利用从入侵植物中提取的生物炭,通过吸附去除 CsTiBr3 催化剂,实现了废水的循环利用。这些生物炭是从一些入侵植物中提取的,如 Acrostichum Aureum、Alternanthera bettzickiana、cyclosorus interrupts 和 Quisqualis indica,其中 Acrostichum Aureum 的吸附量最大。使用异丙醇(IPA)、乙二胺四乙酸(EDTA)和硝酸银(AgNO3)进行的清除剂研究表明,空穴控制着光催化机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Purification of textile wastewater by collective degradation using nanorods of cesium titanium bromide (CsTiBr3) perovskite
The present work recommends a sustainable environmentally safe method for the removal of textile dyes in an economically viable way.The wastewater expelled from the textile dying units contains a mixture of dyes. Here we address the challenge of using a single catalyst for the collective photocatalytic degradation of mixture of six dyes and two samples of textile effluents collected from a local dying unit under direct sunlight. The lead-free perovskite of CsTiBr3 nanorods prepared via the solvothermal process is effective in the collective photocatalytic degradation of dye mixture containing toxic dyes like congo red, crystal violet, malachite green, methylene blue, rhodamine, and methyl orange. The six-dye mixture turned colorless within three hours of direct sunlight exposure. The collective photocatalytic degradation ability of CsTiBr3 perovskite nanorods is successfully exploited for the degradation of the textile effluents. The chemical oxygen demand (COD) analysis guarantees the degradation of dye into non-toxic components. The recycling of wastewater is made possible by the removal of CsTiBr3 catalysts through adsorption using biochar derived from invasive plants. The biochars are extracted from invasive plants such as Acrostichum Aureum, Alternanthera bettzickiana, cyclosorus interrupts, and Quisqualis indica in which Acrostichum Aureum showed maximum adsorption. The scavenger studies using isopropyl alcohol (IPA), ethylene diamine tetra acetic acid (EDTA), and silver nitrate (AgNO3) suggest that holes control the photocatalysis mechanism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


