用于低温 NH3-SCO 的银改性 CuO-Fe2O3 复合催化剂
IF 5.7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
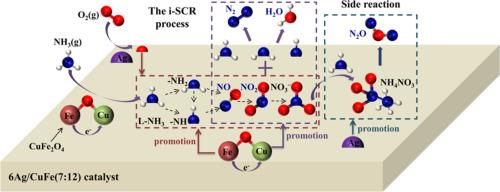
制备了一系列 CuFe 和 Ag 改性 CuFe 复合催化剂,用于氨(NH3-SCO)的低温选择性催化氧化。CuFe(7:12) 催化剂中的铜和铁氧化物在催化 NH3 氧化方面具有显著的协同效应。在 CuFe(7:12) 中添加 6 wt.% 的 Ag 可进一步提高 NH3 转化率和 N2 选择性。200 °C 时,6Ag/CuFe(7:12) 的 NH3 转化率和 N2 选择性分别达到 97.1 % 和 79.3 %。6Ag/CuFe(7:12) 催化剂之所以能在 NH3-SCO 中表现出卓越的性能,除了 Ag 物种的特殊催化作用外,其表面积的增加、CuFe2O4 的形成、Cu 和 Fe 氧化物还原性的改善以及 Cu 和 Fe 的复合和 Ag 的修饰所带来的酸性位点的增加也功不可没。原位 DRIFTS 结果表明,NH3 氧化可能遵循内选择性催化还原机制:NH3 被脱氢并氧化成 NOx 和硝酸盐,形成的 NOx 和硝酸盐被剩余的 NH3、-NH2 和 -NH 还原成 N2 和 H2O。在 CuFe(7:12)上添加 Ag 可抑制 NH3 氧化过程中 NO 和 NO2 的形成,但会促进 N2O 的形成,这可能是由于在 Ag 的存在下 NH4NO3 的形成和分解得到了增强。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ag-modified CuO-Fe2O3 composite catalysts for low-temperature NH3-SCO
A series of CuFe and Ag-modified CuFe composite catalysts were prepared for low-temperature selective catalytic oxidation of ammonia (NH3-SCO). Significant synergistic effects existed between Cu and Fe oxides in the CuFe(7:12) catalyst for catalyzing NH3 oxidation. Loading 6 wt.% Ag over CuFe(7:12) further enhanced NH3 conversion and N2 selectivity. At 200 °C, NH3 conversion and N2 selectivity reached 97.1 % and 79.3 %, respectively, over 6Ag/CuFe(7:12). Besides the exceptional catalytic effects of Ag species, the increased surface area, formation of CuFe2O4, improved reducibility of Cu and Fe oxides, and increased acid sites due to the composite of Cu and Fe and modification with Ag contributed to the superior performance of the 6Ag/CuFe(7:12) catalyst in NH3-SCO. In-situ DRIFTS results showed that NH3 oxidation might follow the internal selective catalytic reduction mechanism: NH3 was dehydrogenated and oxidized to NOx and nitrate species, and the formed NOx and nitrate species were reduced by the remaining NH3, −NH2 and −NH species to N2 and H2O. Loading Ag over CuFe(7:12) suppressed the formation of NO and NO2 but promoted that of N2O during NH3 oxidation, which could be attributed to the enhanced formation and decomposition of NH4NO3 in the presence of Ag.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


