气体水合物相变从形成到分解的热力学和动力学特性及其应用:综述
IF 7.4
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
天然气水合物相变对能源开发、天然气运输和二氧化碳捕集封存领域至关重要。本综述介绍了天然气水合物在相变过程中的特征,包括热力学和动力学特征、解离和重整特征以及促进方法。天然气水合物的相平衡条件决定了其热特性,但今后需要进行一些精确的统计研究,以获得各种相平衡实验结果。此外,由于成核过程的随机性,有必要量化水合物成核因素并提供精确的诱导时间预测模型。在水合物开采应用中,由于温度、压力、抑制剂和传热特性的限制,减压和注热方法可能会受到阻碍。这将导致水合物重整现象,进一步影响水合物开采效率。控制水合物开采过程需要更系统地组合分解方法,并进一步证明记忆效应和纳米气泡等影响因素对水合物重整的作用。此外,还研究了气体水合物相变的促进因素,以实现工业领域水合物的快速形成。搅拌、喷洒、鼓泡方法和添加剂都可用于促进。然而,水合物形成促进过程的能源成本和效率改善问题还需要进一步研究。最后,还提出了一些天然气水合物的应用,包括二氧化碳捕获和封存、天然气储存、海水淡化、冷藏、混合气体分离和污水处理。本综述全面分析了天然气水合物从特征到应用的相变过程,为水合物技术的未来发展提供了参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
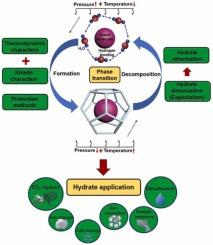
Thermodynamic and kinetic properties of gas hydrate phase transition from formation to decomposition with applications: A review
Gas hydrates phase transition is crucial for energy exploitation, natural gas transportation and CO2 capture storage fields. This review introduces the characteristics of gas hydrate in phase transition process including the thermodynamic and kinetic characters, the dissociation and reformation characters, and the promotion methods. The phase equilibrium conditions of gas hydrate determine its thermal properties, but some accurate statistical studies are needed to obtain a variety of phase equilibrium experimental results in the future. In addition, it is necessary to quantify the factors of hydrate nucleation and provide an accurate induction time prediction model due to the randomness of the nucleation process. In hydrate exploitation applications, the depressurization and heat injection methods may be hindered due to the limitation of temperature, pressure, inhibitor and heat transfer characteristic. It will cause the hydrate reformation phenomenon and further influence the hydrate exploitation efficiency. Controlling the hydrate exploitation process requires a more systematic combination of decomposition methods, and further proving the influence factors such as memory effect and nanobubbles for the hydrate reformation. Besides, the promotion for gas hydrate phase transition also has been studied to make the hydrate rapid formation in the industrial fields come true. Stirring, spraying, bubbling method and additives are available for the promotion. However, the energy cost and efficiency improvement of hydrate formation promotion process need to be further studied. At last, several gas hydrate applications are proposed, including CO2 capture and sequestration, natural gas storage, seawater desalination, cold storage, mixed gas separation and sewage treatment. This review presents an overall analysis of gas hydrate phase transition from characteristics to applications and contributes a reference for future development in hydrate technology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Environmental Chemical Engineering
Environmental Science-Pollution
CiteScore
11.40
自引率
6.50%
发文量
2017
审稿时长
27 days
期刊介绍:
The Journal of Environmental Chemical Engineering (JECE) serves as a platform for the dissemination of original and innovative research focusing on the advancement of environmentally-friendly, sustainable technologies. JECE emphasizes the transition towards a carbon-neutral circular economy and a self-sufficient bio-based economy. Topics covered include soil, water, wastewater, and air decontamination; pollution monitoring, prevention, and control; advanced analytics, sensors, impact and risk assessment methodologies in environmental chemical engineering; resource recovery (water, nutrients, materials, energy); industrial ecology; valorization of waste streams; waste management (including e-waste); climate-water-energy-food nexus; novel materials for environmental, chemical, and energy applications; sustainability and environmental safety; water digitalization, water data science, and machine learning; process integration and intensification; recent developments in green chemistry for synthesis, catalysis, and energy; and original research on contaminants of emerging concern, persistent chemicals, and priority substances, including microplastics, nanoplastics, nanomaterials, micropollutants, antimicrobial resistance genes, and emerging pathogens (viruses, bacteria, parasites) of environmental significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


