增强轻金属分散氢化硼单层的储氢性能
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
通过第一原理计算,我们探索了氢化硼(BH)储存氢气(H2)的潜力。锂(Li)和钾(K)掺杂物增强了氢化硼的氢气存储能力。锂和钾的结合能分别为-2.65 和-1.69 eV,表明它们具有很强的结合力。在 400 K 温度下进行的 Ab initio 分子动力学(AIMD)模拟有助于深入了解掺锂和掺钾 BH 的热稳定性。值得注意的是,H2 分子吸附在金属掺杂的 BH 上会产生很大的结合能,锂和 K 的结合能分别为 -0.45 和 -0.29 eV/H2。在分层吸附的情况下,BH-4Li(BH-4K)可吸附多达 38 个 H2(34 个 H2)分子,其重量密度高达 26.46 (16.57) wt.%,令人印象深刻。即使是在 BH-4Li 和 BH-4K 上的单层 H2 也分别相当于 11.70 wt% 和 7.56 wt%。在应力和应变的影响下,可以调整 H2 的吸附机制。此外,基于 Langmuir 模型的热力学分析被用来阐明在实际温度和压力条件下的 H2 储存能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
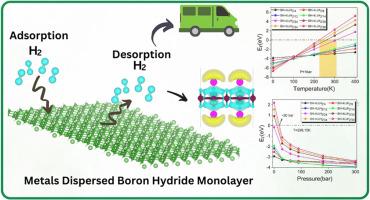
Enhanced hydrogen storage properties of light metals dispersed boron hydride monolayer
Using first-principles calculations, we explore the potentials of boron hydride (BH) for hydrogen (H2) storage. Lithium (Li) and potassium (K) dopants enhance the H2 storage capabilities of BH. The binding energies of Li, and K are found as −2.65 and −1.69 eV, respectively, indicating a strong binding. Ab initio molecular dynamic (AIMD) simulations at 400 K provide insights into the thermal stability of Li-, and K-doped BH. Notably, H2 molecule adsorptions on metal-decorated BH result in substantial binding energies of −0.45 and −0.29 eV/H2 for Li, and K, respectively. Under layered adsorption, the BH–4Li (BH–4K) accommodates up to 38H2 (34H2) molecules, boasting an impressive gravimetric density of 26.46 (16.57) wt.%. Even a single layer of H2 over BH–4Li, and BH–4K corresponds to 11.70 wt% and 7.56 wt%, respectively. Adsorption mechanism of H2 could be tuned under the influence of stress and strain. Additionally, thermodynamic analysis based on Langmuir model is employed to elucidate the H2 storage capabilities under practical conditions of temperature and pressure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


