从设计到 3D 打印:通过双挤压熔融长丝制造技术打印多单元颗粒系统 (MUPS) 的概念验证研究
IF 5.2
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
MUPS(多单位颗粒系统)是一种由小颗粒组成的口服剂型,这些小颗粒被填充进胶囊或压制成片剂。与单片缓释片相比,MUPS 片剂在胃内迅速崩解,释放出所含的小颗粒,可从胃中排出,不受胃肠蠕动的影响。通过调整颗粒成分可以实现释放控制。尽管 MUPS 具有诸多优势,但目前市场上的制剂数量有限。与传统的片剂生产方式相比,3D 打印是生产 MUPS 片剂的一种新的有利方法。由于个性化医疗的研究兴趣日益浓厚,特别是在剂量调整方面,这种灵活的生产方法可能是一个很有前景的概念。因此,本作品提出了一种使用双挤压熔融长丝制造三维打印机打印 MUPS 片剂的概念。总体思路是,两个打印头可以独立使用,第一个打印头打印水溶性片剂外壳,第二个打印头在每个步骤中使用不同的材料将功能性颗粒融入片剂外壳。本研究提出了一种模块化四颗粒层片剂计算机模型,其中包含 196 个直径为 1.4 毫米、高度为 1.0 毫米的圆柱形颗粒,片剂总尺寸为 22.6 × 8.5 × 6.0 毫米。使用无药物成分的市售聚乳酸长丝制作颗粒,使用聚乙烯醇长丝制作片剂外壳,首次概念验证研究揭示了成功打印拟议计算机模型的关键参数(如长丝回缩、Z 偏移和长丝含水量)。此外,还对成功打印的 3D-MUPS 模型药片和加入的颗粒进行了表征,揭示了可重复的制造过程。打印出的模型颗粒直径为 1.27 ± 0.04 毫米,高度为 1.05 ± 0.01 毫米。这种新方法面临的挑战之一是如何避免在使用两个打印头进行打印的过程中由于重熔过程而导致颗粒团聚。在 196 个打印颗粒中,57.54 ± 18.59 % 为单个颗粒。最后,还成功测试了颗粒与聚乙烯醇片剂外壳之间的可转移性和与负载原料药(扑热息痛)的羟丙基甲基纤维素长丝的适用性。平均而言,80% 的扑热息痛在 3 小时(2-4 小时)内释放。总之,这项工作为剂量可调的个性化 MUPS 片剂展示了一种创新的新生产方法,同时也考虑了不同生产工艺带来的新挑战。本文章由计算机程序翻译,如有差异,请以英文原文为准。
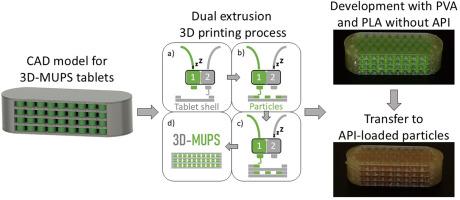
From design to 3D printing: A proof-of-concept study for multiple unit particle systems (MUPS) printed by dual extrusion fused filament fabrication
MUPS (multiple unit particle systems) are oral dosage forms consisting of small particles which are filled into capsules or compressed into tablets. Compared to monolithic sustained-release tablets, MUPS tablets rapidly disintegrate inside the stomach releasing the contained small particles, which can be emptied from the stomach independent of housekeeping waves. Control of release can be achieved by adapting the particle composition. Despite the advantages of MUPS, only a limited number of preparations are available on the market. 3D printing could be a new advantageous method to produce MUPS tablets compared to the conventional production via tableting. Due to the increasing research interest in personalised medicine, especially regarding dose adjustments, this flexible production approach could be a promising concept. Therefore, this work proposes a concept for printing MUPS tablets using a dual extrusion fused filament fabrication 3D printer. The general idea is that the two print heads can be used independently to print a water-soluble tablet shell with the first print head and incorporate functional particles into the tablet shell with a second print head using different materials for each step. In this study, a modular four-particle-layered tablet computer model containing 196 cylindrical particles with a diameter of 1.4 mm, a height of 1.0 mm and a total tablet size of 22.6 × 8.5 × 6.0 mm is proposed. A first proof-of-concept study with drug-free commercially available polylactic acid filament for the particles and polyvinyl alcohol filament for the tablet shell revealed critical parameters (such as filament retraction, z-offset and water content of filaments) for the successful printing of the proposed computer model. In addition, the successfully printed model 3D-MUPS tablets and incorporated particles were characterised, revealing a reproducible manufacturing process. The printed model particles had a diameter of 1.27 ± 0.04 mm and a height of 1.05 ± 0.01 mm. One of the challenges of the new approach was to avoid particle agglomeration because of remelting processes during the printing with two print heads. 57.54 ± 18.59 % of the 196 printed particles were present as single particles. Finally, the transferability and suitability with a model API-loaded (paracetamol) hydroxypropyl methylcellulose filament for the particles and a polyvinyl alcohol tablet shell was successfully tested. On average, 80 % of paracetamol was released within 3 h (2–4 h). Overall, this work shows an innovative new manufacturing method for dose-adjustable personalised MUPS tablets but also considers new challenges arising from the different manufacturing processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Pharmaceutics: X
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
6.60
自引率
0.00%
发文量
32
审稿时长
24 days
期刊介绍:
International Journal of Pharmaceutics: X offers authors with high-quality research who want to publish in a gold open access journal the opportunity to make their work immediately, permanently, and freely accessible.
International Journal of Pharmaceutics: X authors will pay an article publishing charge (APC), have a choice of license options, and retain copyright. Please check the APC here. The journal is indexed in SCOPUS, PUBMED, PMC and DOAJ.
The International Journal of Pharmaceutics is the second most cited journal in the "Pharmacy & Pharmacology" category out of 358 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


