生态相关磷酸盐物种诱发的意想不到的纳米级 CeO2 结构转变。
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
本研究调查了 CeO2 纳米粒子 (NPs) 在含磷酸盐环境中的溶解和微观结构转变。研究发现,2 nm 和 5 nm CeO2 NPs 在磷酸盐缓冲溶液中的溶解行为与在 0.01 M NaClO4 溶液中的溶解行为明显不同。通过同步辐射 X 射线衍射分析和 X 射线吸收光谱,研究了 CeO2 NPs 与磷酸盐物种之间的相互作用,发现氧化物转变为钠铈双磷酸盐,铈主要以 Ce(IV)态存在。根据扫描和透射电子显微镜观察,由此形成的 Na-Ce(IV)磷酸盐由纺锤形的纳米晶棒聚集体组成,这可能是在最初的 CeO2 表面吸附磷酸盐阴离子时形成的。配位分布函数分析表明,Na-Ce(IV) 磷酸盐具有三维框架晶体结构,类似于早先报道的 NaTh2(PO4)3,沿 c 轴有包含无序钠原子的大通道。本研究首次详细分析了磷酸盐诱导的 CeO2 NPs 分化和微结构转变,从而形成了磷酸铈(IV)。在自然生态系统中引入 CeO2 NPs 时也可能会发生类似的过程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
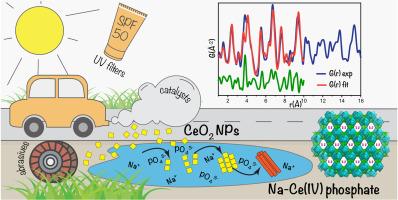
Unexpected nanoscale CeO2 structural transformations induced by ecologically relevant phosphate species
In the present study, the dissolution and microstructural transformation of CeO2 nanoparticles (NPs) in a phosphate-containing milieu were investigated. The dissolution behaviour of 2 nm and 5 nm CeO2 NPs in phosphate buffer solutions was found to differ markedly from that observed in 0.01 M NaClO4. Through synchrotron X-ray diffraction analysis and X-ray absorption spectroscopy, the interaction between CeO2 NPs and phosphate species was examined, revealing the transformation of the oxide into sodium-cerium double phosphate, with cerium predominantly existing in the Ce(IV) state. According to scanning and transmission electron microscopy observations, thus formed Na-Ce(IV) phosphate consists of spindle-like aggregates of nanocrystalline rods, presumably formed during phosphate anions sorption on the initial CeO2 surface. Pair distribution function analysis revealed that Na-Ce(IV) phosphate has a three-dimensional framework crystal structure, similar to NaTh2(PO4)3, as reported earlier, with large channels along the c-axis containing disordered sodium atoms. This study represents the first detailed analysis of phosphate-induced speciation and microstructural transformation of CeO2 NPs, resulting in the formation of Ce(IV) phosphate. Similar processes may occur in natural ecosystems upon the introduction of CeO2 NPs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


