心肌细胞收缩活动对横向和轴向肾小管系统管腔内容物动态的不同影响
IF 4.9
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
摘要
背景:哺乳动物心室心肌细胞有效的兴奋-收缩耦合取决于横轴管系统(TATS),这是一个表面膜内陷网络。TATS 使肌浆膜和肌浆网膜紧密耦合,这对 Ca2+ 诱导的 Ca2+ 快速释放和电刺激下的均匀收缩至关重要。健康心室心肌细胞中的大多数 TATS 由横向小管(TT,约占兔 TATS 的 90%)组成。其余部分主要由轴向小管(AT)组成,其数量较少,研究也较少。然而,在疾病中,TT 和 AT 的相对丰度会发生变化。这种变化的机制和相关性尚不清楚,要了解它们需要更有针对性地研究 AT 结构和功能的动态变化。虽然 TATS 的含量与间质空间是连续的,但它包含在一个扩散受限的区域内。我们之前已经证明,TT 在拉伸和收缩过程中会受到周期性挤压。这可能会导致 TT 成分混合,并加速管腔成分与环境的交换。在此,我们探讨了心肌细胞拉伸和收缩对 AT 的影响:方法:使用三维电子断层扫描技术研究了兔左心室心肌细胞在静止或收缩时的TATS结构和扩散动力学,以及心室组织在静止或拉伸时的TATS含量交换活细胞测量:结果:我们发现:(i) 心肌细胞收缩与 TT 含量表观扩散速度的增加有关,该速度与跳动率和细胞缩短程度成比例。相反,(ii) AT 在收缩过程中出现膜褶皱和收缩,(iii) 收缩对管腔交换动力学没有影响,而(iv) 心肌细胞拉伸与 AT 变直以及 AT 和 TT "挤压 "有关,(v) 支持 AT 和 TT 表观扩散速度的加快。最后,(vi) 我们提出了一个简单的计算模型,概述了 AT 在健康和患病细胞中的潜在相关性:我们的研究结果表明,TT 和 AT 受心脏收缩周期的影响不同,并表明 AT 可能在确保病变心肌细胞中 TATS 网络内容均一性方面发挥作用。需要进一步研究探讨衰竭心肌中不同 TATS 成分的结构和功能重塑的相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
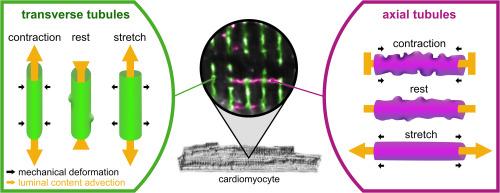
Different effects of cardiomyocyte contractile activity on transverse and axial tubular system luminal content dynamics
Background
Efficient excitation-contraction coupling of mammalian ventricular cardiomyocytes depends on the transverse-axial tubular system (TATS), a network of surface membrane invaginations. TATS enables tight coupling of sarcolemmal and sarcoplasmic reticulum membranes, which is essential for rapid Ca2+-induced Ca2+ release, and uniform contraction upon electrical stimulation. The majority of TATS in healthy ventricular cardiomyocytes is composed of transverse tubules (TT, ∼90 % of TATS in rabbit). The remainder consists of mostly axial tubules (AT), which are less abundant and less well studied. In disease, however, the relative abundance of TT and AT changes. The mechanisms and relevance of this change are not known, and understanding them requires a more targeted effort to study the dynamics of AT structure and function.
While TATS content is continuous with the interstitial space, it is contained within a domain of restricted diffusion. We have previously shown that TT are cyclically squeezed during stretch and contraction. This can contribute to TT content mixing and accelerates luminal content exchange with the environment. Here, we explore the effects of cardiomyocyte stretch and contraction on AT.
Methods
TATS structure and diffusion dynamics were studied using 3D electron tomography of rabbit left ventricular cardiomyocytes, preserved at rest or during contraction, and ventricular tissue preserved at rest or during stretch, as well as live-cell TATS content exchange measurements.
Results
We show (i) that cardiomyocyte contraction is associated with an increase in the apparent speed of diffusion of TT content that scales with beating rate and degree of cell shortening. In contrast, (ii) AT develop membrane folds and constrictions during contraction, (iii) with no effect of contraction on luminal exchange dynamics, while (iv) cardiomyocyte stretch is associated with AT straightening and AT and TT ‘squeezing’ that (v) supports an acceleration of the apparent speed of diffusion in AT and TT. Finally, (vi) we present a simple computational model outlining the potential relevance of AT in healthy and diseased cells.
Conclusions
Our results indicate that TT and AT are differently affected by the cardiac contractile cycle, and suggest that AT may play a role in ensuring TATS network content homogeneity in diseased cardiomyocytes. Further research is needed to explore the interplay of structural and functional remodelling of different TATS components in failing myocardium.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
0.00%
发文量
171
审稿时长
42 days
期刊介绍:
The Journal of Molecular and Cellular Cardiology publishes work advancing knowledge of the mechanisms responsible for both normal and diseased cardiovascular function. To this end papers are published in all relevant areas. These include (but are not limited to): structural biology; genetics; proteomics; morphology; stem cells; molecular biology; metabolism; biophysics; bioengineering; computational modeling and systems analysis; electrophysiology; pharmacology and physiology. Papers are encouraged with both basic and translational approaches. The journal is directed not only to basic scientists but also to clinical cardiologists who wish to follow the rapidly advancing frontiers of basic knowledge of the heart and circulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


