通过跨类型和规模的粉末压制设备转让,加快药用片剂的开发。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
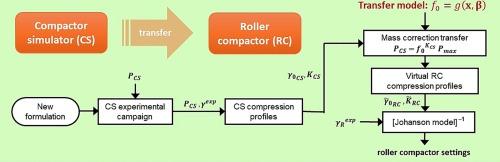
辊子压实是药片生产干法制粒生产线中的一项关键单元操作。在产品开发过程中,人们希望找到所需的辊式压制机(RC)设置,以达到所需的带状固体分数。这些设置可以通过压制粉末混合物的压缩曲线和解释压缩曲线的数学模型来确定。然而,在 RC 中确定压缩曲线需要相对大量的粉末,而这些粉末价格昂贵,在药物开发过程中可能无法获得。作为 RC 的一种经济有效的替代方法,可以使用压制模拟器(CS),这是一种小型设备,使用极少量的粉末来建立压缩轮廓。然而,由于 CS 和 RC 的工作原理不同,因此对于给定的粉末,从这两种设备获得的压缩曲线也不同。在本研究中,我们提出了一种迁移学习方法,该方法可以轻松地根据在 CS 中获得的相同粉末的压缩轮廓来预测给定粉末的 RC 压缩轮廓。基于著名的约翰森模型和质量校正因子理论,我们研究了六种配方的压实行为,其中两种配方包括活性成分,我们发现质量校正因子与被压实粉末的关系不大。我们开发了一种简单、通用的相关性(转移模型),可将质量校正因子仅作为压实压力的函数进行预测。与使用质量校正系数恒定值的情况相比,通过使用所提出的转移模型,对验证粉末的 RC 压缩曲线的预测得到了显著改善。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Accelerating pharmaceutical tablet development by transfer of powder compaction equipment across types and scales
Roller compaction is a key unit operation in a dry granulation line for pharmaceutical tablet manufacturing. During product development, one would like to find the roller compactor (RC) settings that are required to achieve a desired ribbon solid fraction. These settings can be determined from the compression profile of the powder mixture being compacted and a mathematical model that interprets it. However, establishing compression profiles in an RC requires relatively large amounts of powder, which are expensive and may not be available during drug development. As a cost-effective alternative to an RC, a compactor simulator (CS) can be used, which is a small-scale equipment that uses minimal amounts of powder to build the compression profile. However, since the working principles of a CS and an RC are different, the compression profiles obtained from the two devices for a given powder are also different. In this study, we propose a transfer learning approach that allows the RC compression profile of a given powder to be easily predicted from the compression profile obtained in a CS for the same powder. Based on the well-known Johanson model and on the mass correction factor theory, we examine the compaction behavior of six formulations, two of which including active ingredients, and we find that the mass correction factor does not depend significantly on the powder being compacted. We develop a simple, generalized correlation (transfer model) that allows the mass correction factor to be predicted solely as a function of the pressure at which the compaction is carried out. By using the proposed transfer model, the prediction of the RC compression profiles for the validation powders is significantly improved over the case where a constant value of the mass correction factor is used.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


