DSC 揭示了辅料在冷冻过程中对药用多肽聚集倾向的影响。
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
药用多肽在溶液中容易聚集,因此必须添加合适的赋形剂才能使其稳定。为了研究这种稳定性,通常需要进行冗长且成本高昂的实验。本研究开发了一种基于差示扫描量热法(DSC)的方法,可快速评估辅料对药用多肽聚集的稳定特性。所研究的辅料的稳定特性来自于 Tg'附近的热行为,Tg'是富含辅料的相在冷冻后的玻璃化转变温度,是反复冻融循环的函数。研究了药用肽胰高血糖素与辅料树胶糖和乳糖的组合。除辅料类型外,还改变了肽/辅料的浓度比。事实证明,与树胶糖相比,乳糖能更好地稳定胰高血糖素溶液。一方面,聚合开始的时间可以延后,聚合开始后,聚合动力学会减慢。此外,研究还表明,无论测试的是哪种辅料,辅料与肽的比例越高,胰高血糖素的聚集趋势就越小。本文章由计算机程序翻译,如有差异,请以英文原文为准。
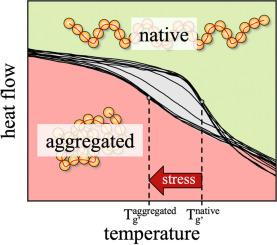
DSC reveals the excipient impact on aggregation propensity of pharmaceutical peptides during freezing
Pharmaceutical peptides are susceptible to aggregation in solution, making stabilization by addition of suitable excipients essential. To investigate this stabilization, lengthy and cost-intensive experiments are often necessary. In this work, a differential scanning calorimetry (DSC) based method was developed that allows a rapid assessment of the stabilization properties of excipients regarding the aggregation of pharmaceutical peptides. The stabilization properties of investigated excipients are derived from the thermal behavior around Tg', the glass-transition temperature of the excipient-rich phase after freezing, as a function of repeated freeze-thaw cycles.
The pharmaceutical peptide glucagon was investigated in combination with the excipients trehalose and lactose. In addition to the type of excipient, the concentration ratio of peptide/excipient was also varied. Lactose proved to better stabilize glucagon solutions compared to trehalose. On the one hand, the onset of aggregation could be delayed and after aggregation started the aggregation kinetics were slowed down. In addition, it was shown that a high excipient to peptide ratio, regardless of the type of excipient tested, reduces the aggregation tendency of glucagon.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


