黄嘌呤氧化酶抑制剂非布索坦对硫代乙酰胺诱导的大鼠肝损伤的保护作用:Nrf2/ HO-1 和 TLR4/ NF-κB 通路的作用。
IF 3.9
3区 医学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
硫代乙酰胺(TAA)是一种典型的肝脏毒性化学物质,可通过氧化应激和炎症诱导造成器官损伤。本研究考察了非布索坦(一种黄嘌呤氧化酶抑制剂;非布,10-15 毫克/千克,口服)对TAA(500 毫克/千克,静注)诱导的大鼠肝损伤的影响。非布能明显减轻 TAA 引起的肝功能参数变化,此外,它还能促进肝脏抗氧化作用,明显提高血红素氧化酶-1(HO-1)、核因子红细胞 2 相关因子 2(Nrf2)、还原型谷胱甘肽(GSH)和超氧化物歧化酶(SOD)的水平,降低肝脏丙二醛(MDA)含量。此外,非布还能提高抗炎细胞因子白细胞介素(IL-10)的水平,降低促炎细胞因子(核因子卡巴B(NF-κB)、IL-1β、高迁移率基团框1(HMGB1))的水平,从而改善肝脏的抗炎状态、此外,非布还能抑制肿瘤坏死因子α(TNF-α)和 IL-6 蛋白及 mRNA 表达水平的升高、TNF-α 和活化的丝裂原活化蛋白激酶(p-JNK/ p-p38 MAPK)的肝脏表达。从组织病理学角度来看,非布能明显使 TAA 引起的肝脏切片改变正常化。总之,非布能通过增强抗氧化能力和减少炎症信号,以剂量依赖性的方式改善 TAA 诱导的肝毒性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
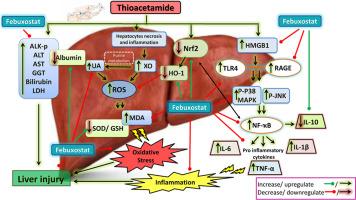
Hepatoprotective effects of the xanthine oxidase inhibitor Febuxostat against thioacetamide-induced liver injury in rats: The role of the Nrf2/ HO-1 and TLR4/ NF-κB pathways
Experimental models of liver injury have been established utilizing thioacetamide (TAA), a classic liver toxic chemical that causes organ damage via oxidative stress and inflammatory induction. This study examined the impact of Febuxostat (a xanthine oxidase inhibitor; Febu, 10–15 mg/kg, orally) against TAA (500 mg/kg, i.p.) -induced liver injury in rats.
Febu significantly attenuated TAA-induced alterations in liver function parameters, in addition to promoting hepatic antioxidant effects through a significant elevation of Heme-oxygenase-1(HO-1), nuclear factor erythroid 2-related factor2 (Nrf2), reduced glutathione (GSH) and superoxide dismutase (SOD) levels and reduction in hepatic malondialdehyde (MDA) content. Moreover, Febu improved the hepatic anti-inflammatory status by increasing the anti-inflammatory cytokine Interleukin (IL-10) level and reducing the levels of the pro-inflammatory cytokines (Nuclear factor kappa B (NF-κB), IL-1β, high-mobility group box1 (HMGB1), receptor for advanced glycation end products (RAGE), and toll-like receptor4 (TLR4) levels, in addition to suppressing the increased protein and mRNA expression levels of tumor necrosis factor alpha (TNF-α) and IL-6, hepatic expression of TNF-α and activated mitogen-activated protein kinases (p-JNK/p-p38 MAPK). Histopathologically, Febu markedly normalized TAA-induced alteration in liver sections. In conclusion, Febu, in a dose-dependent fashion, refines TAA-induced hepatotoxicity by enhancing antioxidant capabilities and decreasing inflammatory signals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food and Chemical Toxicology
工程技术-毒理学
CiteScore
10.90
自引率
4.70%
发文量
651
审稿时长
31 days
期刊介绍:
Food and Chemical Toxicology (FCT), an internationally renowned journal, that publishes original research articles and reviews on toxic effects, in animals and humans, of natural or synthetic chemicals occurring in the human environment with particular emphasis on food, drugs, and chemicals, including agricultural and industrial safety, and consumer product safety. Areas such as safety evaluation of novel foods and ingredients, biotechnologically-derived products, and nanomaterials are included in the scope of the journal. FCT also encourages submission of papers on inter-relationships between nutrition and toxicology and on in vitro techniques, particularly those fostering the 3 Rs.
The principal aim of the journal is to publish high impact, scholarly work and to serve as a multidisciplinary forum for research in toxicology. Papers submitted will be judged on the basis of scientific originality and contribution to the field, quality and subject matter. Studies should address at least one of the following:
-Adverse physiological/biochemical, or pathological changes induced by specific defined substances
-New techniques for assessing potential toxicity, including molecular biology
-Mechanisms underlying toxic phenomena
-Toxicological examinations of specific chemicals or consumer products, both those showing adverse effects and those demonstrating safety, that meet current standards of scientific acceptability.
Authors must clearly and briefly identify what novel toxic effect (s) or toxic mechanism (s) of the chemical are being reported and what their significance is in the abstract. Furthermore, sufficient doses should be included in order to provide information on NOAEL/LOAEL values.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


