G 蛋白偶联雌激素受体激活可减轻 C57BL/6 小鼠顺铂诱导的 CKD:洞察性别差异
IF 3.9
3区 医学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
顺铂治疗后慢性肾脏病(CKD)的发病率和进展与性别差异有关。本研究旨在探讨G1化合物(一种GPER激动剂)对减轻顺铂诱导的慢性肾脏病的潜在作用。为了诱导雄性小鼠、完整雌性小鼠和卵巢切除(OVX)小鼠的慢性肾功能衰竭,通过注射两个周期的 2.5 毫克/千克顺铂(两个周期之间有 16 天的恢复期)来诱导慢性肾功能衰竭。每天注射 G1(50 或 100 μg/kg),持续 6 周。与雌性动物相比,雄性动物的肾损伤更为严重。有趣的是,卵巢切除术导致的肾损伤与雄性相比不明显,但明显高于雌性。血清肌酐的降低、肌酐清除率的升高、NO的产生以及ET1的减少都证明了G1能改善肾功能和血流量。这种肾脏保护作用可归因于其受 TGF-β 调节的免疫调节作用,该作用使平衡转向有利于抗炎细胞因子的产生(IL-10 增加),而不是有利于促炎细胞因子的产生(Th17 表达减少)。减少 TGF-β 的激活还能抑制上皮细胞向间质转化,最终改善慢性肾脏病的发展。通过上调 Nrf2 和随后的抗氧化酶,证明了 G1 的抗氧化潜力。这些数据表明,G1 是一种很有前景的治疗工具,可减轻 CP 引起的 CKD。本文章由计算机程序翻译,如有差异,请以英文原文为准。
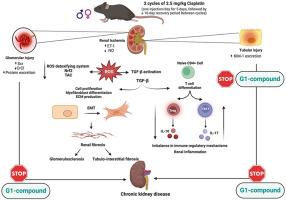
G protein-coupled estrogen receptor activation attenuates cisplatin-induced CKD in C57BL/6 mice: An insight into sex-related differences
Gender contributes to differences in incidence and progression of chronic kidney disease (CKD) post-cisplatin therapy. This study aims at investigating the potential effect of G1 compound, a GPER agonist, on attenuating cisplatin-induced CKD. To induce CKD in male, intact female, and ovariectomized (OVX) mice, CKD was induced by injecting two cycles of 2.5 mg/kg cisplatin with a 16-day recovery period between cycles). G1 (50 or 100 μg/kg was administered daily for 6 weeks. Severity of renal damage was more pronounced in males than females. Interestingly, OVX resulted in renal damage that is non-significant compared to males and significantly higher than females. G1 improved renal function and blood flow as evidenced by reduction of serum creatinine and elevation of creatinine clearance, NO production, and reduction of ET1. This renoprotective effect could be attributed to its immunomodulatory effect regulated by TGF-β that shifted the balance to favor anti-inflammatory cytokine production (increased IL-10) rather than pro-inflammatory cytokines (decreased Th17 expression). Reduction of TGF-β activation also inhibited epithelial-to-mesenchymal transition that eventually ameliorated CKD development. Antioxidant potential of G1 has been demonstrated by upregulation of Nrf2 and subsequent antioxidant enzymes. These data suggest that G1 could be a promising therapeutic tool to attenuate CP-induced CKD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food and Chemical Toxicology
工程技术-毒理学
CiteScore
10.90
自引率
4.70%
发文量
651
审稿时长
31 days
期刊介绍:
Food and Chemical Toxicology (FCT), an internationally renowned journal, that publishes original research articles and reviews on toxic effects, in animals and humans, of natural or synthetic chemicals occurring in the human environment with particular emphasis on food, drugs, and chemicals, including agricultural and industrial safety, and consumer product safety. Areas such as safety evaluation of novel foods and ingredients, biotechnologically-derived products, and nanomaterials are included in the scope of the journal. FCT also encourages submission of papers on inter-relationships between nutrition and toxicology and on in vitro techniques, particularly those fostering the 3 Rs.
The principal aim of the journal is to publish high impact, scholarly work and to serve as a multidisciplinary forum for research in toxicology. Papers submitted will be judged on the basis of scientific originality and contribution to the field, quality and subject matter. Studies should address at least one of the following:
-Adverse physiological/biochemical, or pathological changes induced by specific defined substances
-New techniques for assessing potential toxicity, including molecular biology
-Mechanisms underlying toxic phenomena
-Toxicological examinations of specific chemicals or consumer products, both those showing adverse effects and those demonstrating safety, that meet current standards of scientific acceptability.
Authors must clearly and briefly identify what novel toxic effect (s) or toxic mechanism (s) of the chemical are being reported and what their significance is in the abstract. Furthermore, sufficient doses should be included in order to provide information on NOAEL/LOAEL values.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


