中风后认知障碍中的胼胝体形态改变。
IF 2.7
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
摘要
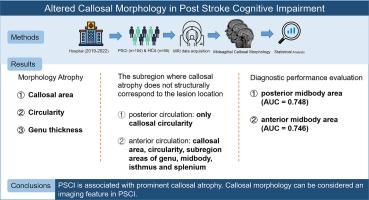
中风是导致死亡和认知障碍的第二大原因。脑卒中后认知障碍(PSCI)是脑卒中幸存者最常见的后遗症之一,但其潜在的神经机制在很大程度上仍不清楚。胼胝体(CC)在半球间整合和半球分离中起着至关重要的作用,CC形态学的变化可能与PSCI的范围重叠。本研究旨在探讨CC的形态学变化及其在PSCI患者中的诊断价值。研究收集了104名PSCI患者和54名人口统计学匹配的健康对照者的结构磁共振成像、神经行为和临床数据。在PSCI患者中观察到CC面积、圆度和真皮厚度显著减少,这些变化与整体认知功能密切相关。亚组分析显示,当病变位于后循环时,CC的圆度明显下降,而当病变位于前循环时,CC面积和圆度均明显下降。接收者操作特征分析表明,CC的中体区域具有很高的诊断价值,其曲线下面积值分别为0.748和0.746。进一步的验证分析表明,这些CC亚区的跨胼胝体纤维与前运动、背侧注意和顶叶前部系统相连。这些研究结果表明,PSCI 患者的 CC 肢体萎缩,尤其是在与前运动皮层和额顶叶网络有跨胼胝体连接的区域,与整体认知障碍并行。这表明,CC形态学可作为诊断和预后PSCI的潜在影像标记。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Altered callosal morphology in post-stroke cognitive impairment
Stroke is the second leading cause of death and cognitive impairment. Post-stroke cognitive impairment (PSCI) is one of the most common sequelae among stroke survivors, yet its underlying neural mechanisms remain largely unclear. The corpus callosum (CC) plays a crucial role in interhemispheric integration and hemispheric segregation, with changes in CC morphology potentially overlapping with the spectrum of PSCI. This study aimed to investigate the morphological changes in the CC and their diagnostic value in PSCI patients. Structural MRI, neurobehavioral, and clinical data were collected from 104 PSCI patients and 54 demographically matched healthy controls. Significant reductions in CC area, circularity, and genu thickness were observed in PSCI patients, with these changes strongly correlating with global cognitive function. Subgroup analysis revealed that CC circularity significantly decreased when lesions were located in the posterior circulation, while both CC area and circularity markedly decreased with anterior circulation lesions. Receiver Operating Characteristic analyses identified the midbody areas of the CC as having high diagnostic value, with area under the curve values of 0.748 and 0.746, respectively. Further validation analyses suggest that the transcallosal fibers in these CC subregions are connected to the premotor, dorsal attention, and frontoparietal system. These findings show that areal CC atrophy in PSCI patients, particularly in regions with transcallosal connections to the premotor cortex and frontoparietal network, parallels global cognitive impairment. This suggests that CC morphology may serve as a potential imaging marker for the diagnosis and prognosis of PSCI.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


