治疗性单克隆抗体的全身和局部用药新策略。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
单克隆抗体(mAbs)是一类不断发展的生物制药,在其发展的各个阶段都取得了明显的进步。本文旨在总结 mAbs 给药方面的新策略。目前,mAb 可通过肠外给药途径进行全身给药,重点是皮下给药。此外,为了方便患者治疗,还探索了其他侵入性较小的 mAbs 给药途径,如吸入、鼻腔、透皮和口服给药。文献数据显示,通过吸入、鼻腔、透皮、瘤内、玻璃体内和阴道给药等途径局部给药的 mAbs 具有较高的疗效和较少的全身不良反应。然而,到目前为止,只有用于玻璃体内给药的 mAb 药物,这主要是由于生物利用度较高,而鼻内喷雾剂已被批准为医疗器械。本综述强调了在批准新型给药途径(可能是通过吸入)方面的有希望的数据,但考虑到目前存在的障碍,进一步的深入研究是必不可少的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
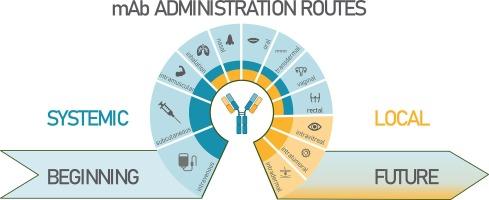
Novel strategies in systemic and local administration of therapeutic monoclonal antibodies
Monoclonal antibodies (mAbs) are an evolving class of biopharmaceuticals, with advancements evident across various stages of their development. While discovery, mAb chemical optimization, production and purification processes have been thoroughly reviewed, this paper aims to offer a summary of novel strategies in administration of mAbs. At present, systemic delivery of mAbs is available through parenteral administration routes with focus on subcutaneous administration. In addition, oriented toward patient-friendly therapy, other less invasive administration routes of mAbs, such as inhalation, nasal, transdermal, and oral administration, are explored. Literature data reveals the potential for local delivery of mAbs via inhalation, nasal, transdermal, intratumoral, intravitreal and vaginal administration, offering high efficacy with fewer systemic adverse effects. However, to date, only mAb medicines are available for intravitreal administration, mainly due to higher bioavailability, and an intranasal spray is authorised as a medical device. The review highlights the promising data in approval of novel administration routes, likely through inhalation, but further intensive research considering the current obstacles, is essential.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


