改变活性位点环路动力学可增强色氨酸合成酶α亚基的独立活性
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
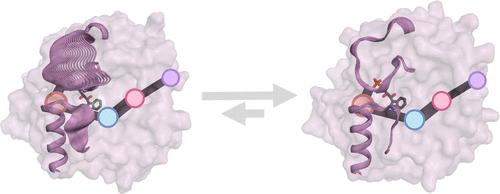
受异位调节的双功能色氨酸合成酶 αβα 酶的α-亚基(TrpA)催化吲哚-甘油磷酸酯(IGP)逆醛醇裂解为 3-磷酸甘油醛(G3P)和吲哚。该酶的活性高度依赖于 β-亚基(TrpB),它通过异构调节和激活 TrpA 来增强功能。这与来自玉米的同源 BX1 酶形成了鲜明对比,后者可以催化与 TrpA 相同的反应,而不需要任何额外的结合伙伴。在这项研究中,我们对同源的 ZmBX1 和 ZmTrpA 酶的构象景观进行了计算评估和比较。我们的研究结果表明,增强 TrpA 的独立活性需要调节两个相关活性位点环路(环路 6 和环路 2)的构象动态,这两个环路需要同步进入催化激活的闭合状态以进行 IGP 裂解,以及进入开放状态以促进吲哚/G3P 的释放。以进化蓝图 ZmBX1 为灵感,利用我们开发的基于相关性的工具最短路径图(shortest path map),我们计算设计了一种名为 ZmTrpASPM4-L6BX1 的变体,该变体在 IGP 的反醛裂解过程中的催化效率提高了 163 倍。这项研究展示了微调活性位点环的构象动态对改变和提高功能的重要性,尤其是在构象变化决定速率的情况下。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Altering Active-Site Loop Dynamics Enhances Standalone Activity of the Tryptophan Synthase Alpha Subunit
The α-subunit (TrpA) of the allosterically regulated bifunctional tryptophan synthase αββα enzyme catalyzes the retro-aldol cleavage of indole-glycerol phosphate (IGP) to d-glyceraldehyde 3-phosphate (G3P) and indole. The activity of the enzyme is highly dependent on the β-subunit (TrpB), which allosterically regulates and activates TrpA for enhanced function. This contrasts with the homologous BX1 enzyme from Zea mays that can catalyze the same reaction as TrpA without requiring the presence of any additional binding partner. In this study, we computationally evaluated and compared the conformational landscapes of the homologous ZmBX1 and ZmTrpA enzymes. Our results indicate that enhanced TrpA standalone activity requires the modulation of the conformational dynamics of two relevant active-site loops, loop 6 and 2, that need to be synchronized for accessing the catalytically activated closed state for IGP cleavage, as well as open states for favoring indole/G3P release. Taking as inspiration the evolutionary blueprint ZmBX1 and using our developed correlation-based tool shortest path map focused on the rate-determining conformational transition leading to the catalytically activated closed state, we computationally designed a variant named ZmTrpASPM4-L6BX1, which displays a 163-fold improvement in catalytic efficiency for the retro-aldol cleavage of IGP. This study showcases the importance of fine-tuning the conformational dynamics of active-site loops for altering and improving function, especially in those cases in which a conformational change is rate determining.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


