用于癌症疗法的微型和增强型 CRISPR 激活剂
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
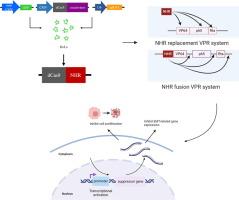
引言RNA引导的核酸酶Cas9可用作可编程转录激活剂,但其在真核生物中的有效性仍有提升空间,其在癌症基因治疗中的潜力也鲜有研究。为了提高 CRISPRa 工具的激活能力,我们将辅助激活子与高效的 dCas9-VPR 系统融合,构建了一个新的激活系统。利用 RNA-seq 评估全基因组的激活特异性。为了评估新构建的激活系统在癌症基因治疗中的价值,我们激活了肿瘤抑制基因 PER2 和 ZNF382 的表达,并进行了癌细胞增殖变化 qRT-PCR 和克隆形成分析。结果在这项研究中,我们筛选了来自秀丽隐杆线虫的 NHR 模块,与 VP64 相比,该模块体积小巧,表现出很高的转录激活能力。我们成功地证明了它在哺乳动物细胞中激活内源基因的效率。此外,我们还开发了一种名为 NHR-VP64-p65-Rta (NVPR)的增强型融合变体,与之前建立的 VPR 模块相比,它显示出更高的效率,使其成为一种有效的 CRISPRa 工具。dCas9-NVPR 复合物在全基因组范围内也表现出高度特异性。最后,我们利用 dCas9-NVPR 工具恢复了肿瘤抑制基因 PER2 和 ZNF382 的表达,有效抑制了癌细胞的恶性表型。这一创新拓展了现有基因编辑工具的范围,进一步验证了基于 CRISPR 的方法在精准医疗中的巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mini and enhanced CRISPR activators for cancer therapies
Introduction
The RNA-guided nuclease Cas9 can be used as a programmable transcription activator, but there is still room for improvement in its effectiveness in eukaryotes, and its potential in cancer genetic therapy has been poorly investigated.Objectives
We aim to construct optimized CRISPRa tools and detect their potential role in cancer therapy by screening 9aa-TAD.Methods
We selected a range of transcriptional coactivators for programmable activation and analyzed their effects on the expression of multiple endogenous genes using Flow cytometry and qRT-PCR. In order to improve the activation capacity of the CRISPRa tool, we fused the coactivators with the efficient dCas9-VPR system to construct a new activation system. Utilize RNA-seq to assess the activation specificity of genome-wide. To evaluate the value of the newly constructed activation system in cancer gene therapy, we activated the expression of the tumor suppressor genes PER2 and ZNF382, and performed changes in cancer cell proliferation qRT-PCR and clonal formation analysis.Results
In this study, we screened the NHR module from C. elegans, which demonstrated a high transcription activation capacity with a compact size compared to VP64. We successfully demonstrated its efficiency in activating endogenous genes in mammalian cells. Furthermore, we developed an enhanced fused variant called NHR-VP64-p65-Rta (NVPR), which showed even higher efficiency compared to the previously established VPR module, making it an effective CRISPRa tool. The dCas9-NVPR complex also exhibited high specificity on a genome-wide scale. Finally, we utilized the dCas9-NVPR tool to restore the expression of tumor suppressor genes PER2 and ZNF382, effectively inhibiting the malignant phenotype of cancer cells.Conclusion
We have successfully developed and demonstrated a breakthrough CRISPRa tool with promising implications for cancer genetic therapy. This innovation expands the range of available gene editing tools and further validates the immense potential of CRISPR-based approaches in precision medicine.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


