揭示腐蚀产物和氧还原对薄电解质层下 Mg-4Nd-0.4Zr 合金腐蚀的相互作用
IF 11.2
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
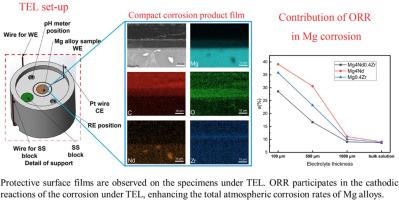
尽管氢进化反应(HER)被认为是镁腐蚀的主要阴极反应,但最近已证实氧还原反应(ORR)是次要的阴极反应。影响镁(Mg)合金氧还原反应的因素尚不清楚,尤其是在电解质层较薄(TEL)的情况下。在这项工作中,研究了腐蚀产物膜对镁合金在薄膜电解质层下和大体积溶液中阴极反应的影响。ORR 不会影响镁合金腐蚀过程中的氢演化率。因此,由于氧气的存在,在 TEL 下通过氢演化试验测量的镁合金腐蚀速率并不准确。而失重试验是评估镁合金在 TEL 下腐蚀速率的一种更准确的方法。经证实,ORR 参与了 Mg-4Nd-0.4Zr、Mg-4Nd 和 Mg-0.4Zr 合金在 TEL 下的腐蚀。在 100μm TEL 中,ORR 在 Mg-4Nd-0.4Zr、Mg-4Nd 和 Mg-0.4Zr 合金腐蚀的阴极反应中的贡献率最高,分别为 28.6%、39.1% 和 35.8%。Mg-4Nd-0.4Zr 合金上的保护膜对氧气的扩散具有更强的抑制作用,从而降低了阴极反应中的 ORR 贡献。此外,研究还表明,制备具有腐蚀产物保护膜的镁合金可以抑制大气中 ORR 引起的腐蚀。这项研究强调了腐蚀产物膜对镁腐蚀中 ORR 的影响,尤其是在 TEL 条件下。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Unveiling the interaction between corrosion products and oxygen reduction on the corrosion of Mg–4Nd–0.4Zr alloy under thin electrolyte layers
Although hydrogen evolution reaction (HER) is considered to be the main cathodic reaction of Mg corrosion, oxygen reduction reaction (ORR) has been recently confirmed to be a secondary cathodic reaction. The factors affecting ORR of magnesium (Mg) alloys are still unclear, especially in cases under thin electrolyte layers (TEL). In this work, the influence of the corrosion product films on the cathodic reactions of Mg alloys under TEL and in a bulk solution was investigated. ORR does not influence the hydrogen evolution rates in the corrosion of Mg alloys. Therefore, with the existence of oxygen, corrosion rates of Mg alloys measured by hydrogen evolution tests are not accurate under TEL. And weight loss test is a more accurate method to evaluate Mg corrosion rates under TEL. ORR was confirmed to participate in the corrosion of Mg–4Nd–0.4Zr, Mg–4Nd and Mg–0.4Zr alloys under TEL. In 100-μm TEL, the highest contribution of ORR in cathodic reactions for the corrosion of Mg–4Nd–0.4Zr, Mg–4Nd and Mg–0.4Zr alloys are 28.6%, 39.1%, and 35.8%, respectively. The more protective film on Mg–4Nd–0.4Zr alloy provides a stronger inhibition effect against the diffusion of oxygen, leading to decreased ORR contribution in cathodic reactions. In addition, it is suggested that the preparation of Mg alloys with protective corrosion product films can inhibit the corrosion induced by ORR in the atmosphere. This work emphasizes the effects of corrosion product films on ORR in Mg corrosion, especially under TEL.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Materials Science & Technology
工程技术-材料科学:综合
CiteScore
20.00
自引率
11.00%
发文量
995
审稿时长
13 days
期刊介绍:
Journal of Materials Science & Technology strives to promote global collaboration in the field of materials science and technology. It primarily publishes original research papers, invited review articles, letters, research notes, and summaries of scientific achievements. The journal covers a wide range of materials science and technology topics, including metallic materials, inorganic nonmetallic materials, and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


