嘧啶和哒嗪化合物配位化学综述:键合、螯合和腐蚀抑制
IF 23.5
1区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
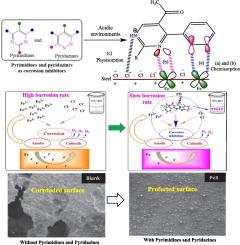
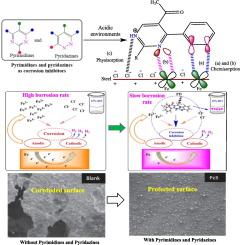
金属老化仍然是众多工业领域面临的严峻挑战,因此需要不断努力寻找有效、可持续和无毒的化学抑制剂。嘧啶和哒嗪属于一类杂环化合物,由于其化学结构多变、保护性能良好,作为潜在的缓蚀剂受到了广泛关注。值得注意的是,嘧啶(C4H4N2)、哒嗪(C4H4N2)及其衍生物的六元杂环中的氮原子因能与金属表面形成配位键而闻名。嘧啶、哒嗪及其衍生物通过在金属表面的吸附作用形成腐蚀抑制性疏水层。π电子的广泛共轭增强了疏水膜的耐久性和功效。在不同的金属/电解质体系中,它们在低浓度(1 mM)时的抑制效率从 70% 到 100% 不等。本综述概述了这些杂环化合物在螯合和配位方面的特性和应用。此外,还全面介绍了它们作为水相抑制剂在不同金属/电解质体系中的潜在应用。利用实验和计算工具,重点研究了嘧啶和哒嗪的配位化学,并强调了它们在不同腐蚀环境中防止金属降解的吸附行为。最后,介绍了有关嘧啶和哒嗪有效性的专利文献以及未来展望。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A critical review of coordination chemistry of pyrimidine and pyridazine compounds: Bonding, chelation and corrosion inhibition
Metallic deterioration remains a formidable challenge in numerous industrial sectors, necessitating the continuous, intense search for effective, sustainable and non-toxic chemical inhibitors. Pyrimidines and pyridazines belong to a class of heterocycles that have garnered significant attention as potential corrosion inhibitors due to their versatile chemical configuration and promising protection performances. Notably, the nitrogen atoms in the six-membered heterocyclic ring of pyrimidine (C4H4N2), pyridazine (C4H4N2), and their derivatives are well known for their capacity to form coordination bonds with metal surfaces. Pyrimidine, pyridazine, and their derivatives form corrosion-inhibitive hydrophobic layers through their adsorption on the metal surfaces. The widespread conjugation of π-electrons enhances the durability and efficacy of the hydrophobic film. They demonstrate excellent inhibition efficiencies ranging from 70 to 100 % at low concentrations (<1 mM) for different metal/electrolyte systems. This review provides an overview of the properties and application of these heterocyclic compounds in chelation and coordination. Furthermore, their potential applications as aqueous phase inhibitors for different metal/electrolyte systems were comprehensively covered. Using experimental and computational tools, emphasis was placed on the coordination chemistry of pyrimidine and pyridazine, and its adsorption behaviour against metallic degradation in diverse corrosive environments was highlighted. Finally, patent literature on the effectiveness of pyrimidine and pyridazine and future perspectives were presented.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Coordination Chemistry Reviews
化学-无机化学与核化学
CiteScore
34.30
自引率
5.30%
发文量
457
审稿时长
54 days
期刊介绍:
Coordination Chemistry Reviews offers rapid publication of review articles on current and significant topics in coordination chemistry, encompassing organometallic, supramolecular, theoretical, and bioinorganic chemistry. It also covers catalysis, materials chemistry, and metal-organic frameworks from a coordination chemistry perspective. Reviews summarize recent developments or discuss specific techniques, welcoming contributions from both established and emerging researchers.
The journal releases special issues on timely subjects, including those featuring contributions from specific regions or conferences. Occasional full-length book articles are also featured. Additionally, special volumes cover annual reviews of main group chemistry, transition metal group chemistry, and organometallic chemistry. These comprehensive reviews are vital resources for those engaged in coordination chemistry, further establishing Coordination Chemistry Reviews as a hub for insightful surveys in inorganic and physical inorganic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


