可单独控制 BMP 和 FGF 释放以调节骨再生的纳米纤维三维支架。
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
目前,骨形态发生蛋白(BMPs)的临床应用仅限于少数特定适应症。在局部控制下释放生长因子组合是改善基于 BMP 的骨修复的一种很有前景的策略。然而,这种方法的成功需要开发一种有效的释放系统,并正确选择能增强 BMP 活性的生长因子。碱性成纤维细胞生长因子(bFGF,又称 FGF-2)已显示出促进骨修复的前景,但报道的结果却相互矛盾。考虑到 FGF-2 复杂的生物活性,我们假设只有对两种因子的剂量和动力学参数进行单独定制,FGF-2 才能促进 BMP 诱导的骨再生。在本研究中,我们对细胞增殖、分化和矿化对因子剂量、给药模式(连续或同步)和释放速率的反应进行了系统的体外研究。随后,我们设计了可单独控制 BMP-7 和 FGF-2 释放的聚乳酸-聚乙二醇(PLGA)纳米球,并将其附着在聚乳酸(PLLA)纳米纤维支架上。数据显示,在体内骨再生模型中,以较快的释放速度提供相对较高的 FGF-2 剂量(100 ng/scaffold),或以较慢的释放速度提供相对较低的 FGF-2 剂量(10 ng/scaffold),都能加速 BMP-7 诱导的骨形成。相比之下,高剂量的 FGF-2(1000 纳克/手镯)在所有条件下都会抑制骨再生。体外和体内数据表明,FGF-2通过协调FGF-2剂量和释放动力学,促进干细胞迁移、增殖和血管生成,从而改善BMP-7诱导的骨再生。意义说明:骨形态发生蛋白(BMPs)是骨发育和再生过程中最有效的生长/分化因子。然而,由于目前的 BMP 产品所需的超生理剂量及其副作用,BMP 的临床应用仅限于少数特定适应症。人们非常希望通过局部控制 BMP 的给药和额外的生长因子来增强其成骨作用。然而,不同的生长因子具有不同的作用机制。在此,我们报告了一种纳米纤维支架,它在尺寸和几何形状上模仿胶原蛋白,并固定有可生物降解的纳米球,以实现 BMP7 和 FGF2 的局部和独特释放。系统研究表明,具有不同时间释放曲线的低剂量 BMP7 和 FGF2 能以最佳方式促进骨再生。本文章由计算机程序翻译,如有差异,请以英文原文为准。
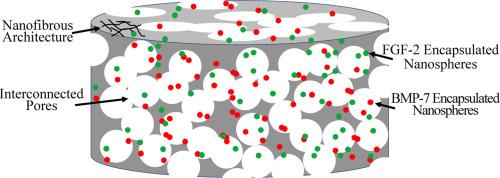
Nanofibrous 3D scaffolds capable of individually controlled BMP and FGF release for the regulation of bone regeneration
The current clinical applications of bone morphogenetic proteins (BMPs) are limited to only a few specific indications. Locally controlled delivery of combinations of growth factors can be a promising strategy to improve BMP-based bone repair. However, the success of this approach requires the development of an effective release system and the correct choice of growth factors capable of enhancing BMP activity. Basic fibroblast growth factor (bFGF, also known as FGF-2) has shown promise in promoting bone repair, although conflicting results have been reported. Considering the complex biological activities of FGF-2, we hypothesized that FGF-2 can promote BMP-induced bone regeneration only if the dosage and kinetic parameters of the two factors are individually tailored. In this study, we conducted systematic in vitro studies on cell proliferation, differentiation, and mineralization in response to factor dose, delivery mode (sequential or simultaneous), and release rate. Subsequently, we designed individually controlled BMP-7 and FGF-2 release poly(lactide-co-glycolide) (PLGA) nanospheres attached to the poly(l-lactic acid) (PLLA) nanofibrous scaffolds. The data showed that BMP-7-induced bone formation was accelerated by a relatively higher FGF-2 dose (100 ng/scaffold) delivered at a faster release rate, or by a relatively lower FGF-2 dose (10 ng/scaffold) at a slower release rate in an in vivo bone regeneration model. In contrast, a very high dose of FGF-2 (1000 ng/scaffold) inhibited bone regeneration under all conditions. In vitro and in vivo data suggest that FGF-2 improved BMP-7-induced bone regeneration by coordinating FGF-2 dosage and release kinetics to enhance stem cell migration, proliferation, and angiogenesis.
Statement of significance
Bone morphogenetic proteins (BMPs) are the most potent growth/differentiation factors in bone development and regeneration. However, the clinical applications of BMPs have been limited to only a few specific indications due to the required supraphysiological dosages with the current BMP products and their side effects. Locally controlled delivery of BMPs and additional growth factors that can enhance their osteogenic potency are highly desired. However, different growth factors act with different mechanisms. Here we report a nanofibrous scaffold that mimics collagen in size and geometry and is immobilized with biodegradable nanospheres to achieve local and distinct release profiles of BMP7 and FGF2. Systematic studies demonstrated low dose BMP7 and FGF2 with different temporal release profiles can optimally enhance bone regeneration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


