大鼠背侧后顶叶皮层的注意加工。
IF 1.8
4区 心理学
Q3 BEHAVIORAL SCIENCES
引用次数: 0
摘要
众所周知,人类的后顶叶皮层(PPC)支持持续注意力。具体来说,自上而下的注意通常在背侧区域进行处理,而自下而上的调节则更多地发生在腹侧。然而,在啮齿类动物模型中,目前还不清楚持续注意是否需要顶叶皮层,或者解剖区域之间是否存在类似的功能分离。因此,本研究旨在调查啮齿动物背侧 PPC(dPPC)在持续注意中的贡献。我们使用了五选一连续反应时间任务(5CSRTT),并将神经毒性 dPPC 损伤的大鼠与假手术大鼠进行了比较。我们发现,dPPC 病变的大鼠做出正确选择的准确性较低,所需的时间较长,这表明大鼠的注意力受损,处理速度降低。然而,这种影响仅限于术后测试的最初几天。在明显恢复后,病变组的遗漏率升高,在动机和活动能力没有降低的情况下,这也可以解释为注意力受损。在随后的挑战测试中,病变组做出正确反应的延迟时间普遍延长,表明处理速度下降。在任何时候都没有观察到过早反应或锲而不舍反应的差异,这表明 dPPC 病变不会影响冲动性和强迫性。这种行为模式表明,虽然完整的dPPC支持目标驱动(自上而下)的注意力调节,但它很可能在处理刺激驱动(自下而上)的注意力方面不起核心作用。此外,在缺乏功能完备的 dPPC 的情况下,补偿机制也能支持持续注意,尽管这是以牺牲处理速度为代价的。我们的研究结果证实了啮齿类动物的PPC参与调节持续注意,并为自上而下和自下而上的注意加工之间的功能分离提供了初步证据,从而为相关文献提供了参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
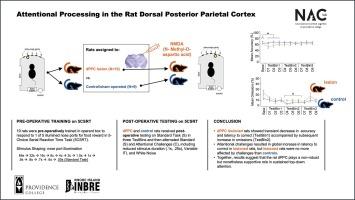
Attentional processing in the rat dorsal posterior parietal cortex
The human posterior parietal cortex (PPC) is known to support sustained attention. Specifically, top-down attention is generally processed in dorsal regions while bottom-up regulation occurs more ventrally. In rodent models, however, it is still unclear whether the PPC is required for sustained attention, or whether there is a similar functional dissociation between anatomical regions. Consequently, the aim of this study was to investigate the contribution of the rodent dorsal PPC (dPPC) in sustained attention. We used the five-choice serial reaction time task (5CSRTT) and compared rats with neurotoxic dPPC lesions to sham operated rats. We found that rats with dPPC lesions were less accurate and took longer to make correct choices, indicating impaired attention and reduced processing speed. This effect, however, was limited to the first few days of post-operative testing. After an apparent recovery, omissions became elevated in the lesion group, which, in the absence of reduced motivation and mobility, can also be interpreted as impaired attention. In subsequent challenge probes, the lesion group displayed globally elevated latency to make a correct response, indicating reduced processing speed. No differences in premature responses or perseverative responses were observed at any time, demonstrating that dPPC lesions did not affect impulsivity and compulsivity. This pattern of behavior suggests that while intact dPPC supports goal-driven (top-down) modulation of attention, it likely does not play a central role in processing stimulus-driven (bottom-up) attention. Furthermore, compensatory mechanisms can support sustained attention in the absence of a fully functioning dPPC, although this occurs at the expense of processing speed. Our results inform the literature by confirming that rodent PPC is involved in regulating sustained attention and providing preliminary evidence for a functional dissociation between top-down and bottom-up attentional processing.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.10
自引率
7.40%
发文量
77
审稿时长
12.6 weeks
期刊介绍:
Neurobiology of Learning and Memory publishes articles examining the neurobiological mechanisms underlying learning and memory at all levels of analysis ranging from molecular biology to synaptic and neural plasticity and behavior. We are especially interested in manuscripts that examine the neural circuits and molecular mechanisms underlying learning, memory and plasticity in both experimental animals and human subjects.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


