黄芪建中煎剂通过调节肠道-甲状腺轴改善大鼠的胃肠化生。
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
背景:胃肠化生(GIM)是胃癌发展过程中的一个关键阶段。黄芪建中煎(HQJZ)已成为中医临床治疗胃肠化生不耐寒患者的主要治疗策略,但其详细机制仍不甚明了:本研究旨在以肠道微生物群-甲状腺轴为基础,阐明 HQJZ 在大鼠模型中缓解 GIM 的分子机制:方法:通过给大鼠注射冷水杨酸和脱氧胆酸钠(SDC)12周,然后灌胃HQJZ治疗4周,建立了GIM大鼠模型。连朴阴(LPY)被用作对比配方。通过耐寒性和温度趋向实验评估了 GIM 大鼠的耐寒特性。甲状腺病理变化通过 HE 染色进行评估,甲状腺功能通过 ELISA 定量 T3 和 T4 水平进行测量。利用 16S rRNA 基因测序分析了肠道微生物群,并利用代谢组学量化了粪便中的丁酸和血清代谢物。在Nthy-ori 3-1细胞模型中验证了关键的分子机制:结果:HQJZ(而非 LPY)能明显改善 GIM 大鼠的胃黏膜和甲状腺组织病变,提高血清中甲状腺激素 T3 和 T4 的水平,增强耐寒能力。HQJZ 治疗能促进粪便中产生丁酸的细菌(特别是 Allobaculum 和双歧杆菌)的富集,从而使粪便中的丁酸浓度明显增加。HQJZ 能明显降低线粒体损伤相关血清代谢物的水平,包括对甲酚硫酸盐和吲哚硫酸盐。体内机理研究进一步表明,丁酸不仅能改善甲状腺组织病变,还能恢复 GIM 大鼠粪便微生物群结构和低温趋向性。此外,丁酸还能减轻SDC对这些细胞线粒体的损伤,这表现在活性氧水平降低、ATP生成增加以及线粒体膜电位提高。重要的是,体外研究显示,丁酸盐通过上调TG、TPO和TSHR的表达,保护Nthy-ori 3-1细胞免受SDC诱导的损伤:结论:HQJZ通过丰富肠道丁酸盐产菌促进GIM大鼠的耐寒能力并改善其甲状腺功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
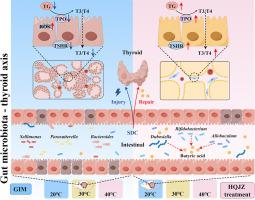
Huangqi Jianzhong decoction improves gastric intestinal metaplasia in rats by regulating the gut‒thyroid axis
Background
Gastric intestinal metaplasia (GIM) is a crucial stage in the progression of gastric cancer. Huangqi Jianzhong decoction (HQJZ) has emerged as a leading therapeutic strategy for treating GIM patients with cold intolerance in traditional Chinese medicine clinics, but the detailed mechanism remains poorly understood.
Objective
The present study aimed to elucidate the molecular mechanism by which HQJZ alleviates GIM in a rat model on the basis of the gut microbiota‒thyroid axis.
Methods
A GIM rat model was established by administering cold salicylic acid and sodium deoxycholate (SDC) for 12 weeks, followed by gavage treatment with HQJZ for an additional four weeks. Lianpu Yin (LPY) was used as a comparison formula. The cold tolerance characteristics of GIM rats were evaluated using cold tolerance and temperature‒tropism experiment experiments. Thyroid pathological changes were evaluated with HE staining, and thyroid function was measured via quantification of T3 and T4 levels with ELISA. The gut microbiota was analyzed using 16S rRNA gene sequencing, and fecal butyric acid and serum metabolites were quantified utilizing metabolomics. The key molecular mechanism was verified in the Nthy-ori 3–1 cell model.
Results
HQJZ, but not LPY, significantly improved gastric mucosa and thyroid tissue lesions in GIM rats, increased the serum levels of the thyroid hormones T3 and T4, and enhanced cold tolerance. HQJZ treatment promoted the enrichment of fecal butyrate-producing bacteria, specifically the bacteria Allobaculum and Bifidobacterium, resulting in a marked increase in fecal butyric acid concentrations. HQJZ treatment significantly diminished the levels of mitochondrial damage-related serum metabolites, including p-cresol sulfate and indoxyl sulfate. Mechanistically, in vivo investigations further demonstrated that butyric acid not only improved thyroid tissue lesions but also restored the fecal microbiota structure, as well as low-temperature tropism, in GIM rats. Furthermore, butyrate diminished the mitochondrial damage induced by SDC in these cells, as evidenced by decreased reactive oxygen species levels and increased ATP production and mitochondrial membrane potential. Importantly, in vitro studies revealed that butyrate protected against SDC-induced injury in Nthy-ori 3–1 cells through the upregulation of TG, TPO, and TSHR expression.
Conclusions
HQJZ promotes cold tolerance and improves thyroid function in GIM rats by enriching gut butyrate-producing bacteria.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


