推进美国食品及药物管理局(FDA)批准的鼻脑给药技术,更好地治疗抑郁症和精神疾病。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
抑郁症和精神疾病的发病率在全球范围内不断上升,尽管美国食品及药物管理局已批准了多种药物,但治疗仍然充满挑战。许多传统的抗抑郁剂和抗精神病制剂都面临着溶解度低、首过代谢率高、生物利用度低、血脑屏障渗透不足以及全身副作用等问题。这些问题导致药效降低、起效较慢以及患者对治疗的依从性下降。为了解决这些问题,最近的研究探索了鼻脑给药途径。这种方法具有多种优势,包括无创给药、直接进入大脑、起效迅速、减少全身暴露和副作用、避免首过代谢、提高生物利用度、精确给药和改善患者依从性。这种方法使用的制剂包括脂质纳米颗粒、聚合物纳米颗粒、鼻凝胶、立方体、niosomes、聚合物胶束、纳米悬浮剂、纳米乳剂、纳米胶囊和弹性体。本综述分析并总结了已发表的有关经 FDA 批准的抗抑郁药物和抗精神病药物从鼻腔向大脑给药的研究成果,重点关注这些纳米制剂的制备、表征、药代动力学、药效学和毒性分析。本文章由计算机程序翻译,如有差异,请以英文原文为准。
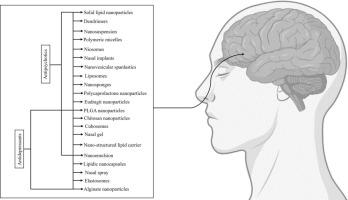
Advancement in the Nose-to-Brain Drug delivery of FDA-approved drugs for the better management of Depression and Psychiatric disorders
The Prevalence of Depressive and Psychiatric disorders is increasing globally, and despite the availability of numerous FDA-approved drugs, treatment remains challenging. Many conventional antidepressants and antipsychotic formulations face issues such as low solubility, high first-pass metabolism, poor bioavailability, inadequate blood–brain barrier penetration, and systemic side effects. These challenges lead to reduced efficacy, slower onset of action, and decreased patient adherence to treatment. To address these problems, recent studies have explored the nose-to-brain route for drug delivery. This method offers several advantages, including non-invasive drug administration, direct access to the brain, rapid onset of action, reduced systemic exposure and side effects, avoidance of first-pass metabolism, enhanced bioavailability, precision dosing, and improved patient compliance. The formulations used for this approach include lipidic nanoparticles, polymeric nanoparticles, nasal gels, cubosomes, niosomes, polymeric micelles, nanosuspensions, nanoemulsions, nanocapsules, and elastosomes. This review analyzes and summarizes the published work on the nose-to-brain delivery of FDA-approved antidepressants and antipsychotic drugs, with a focus on the preparation, characterization, pharmacokinetics, pharmacodynamics, and toxicity profiling of these nanoformulations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


