DigiChemTree 实现了可编程光诱导碳烯生成,可按需进行化学合成。
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
从文献或数据库中获取化学反应规程时,化学反应的可重复性对学术界、工业界专业人士,甚至现在的机器学习过程都是极大的挑战。要在光化学条件下合成有机分子,需要花费数年时间进行反应优化、高技能的人力、较长的反应时间等,导致成本难以承受,研发工作进展缓慢。在此,我们引入了以人工智能为支撑的 DigiChemTree,以自动优化光化学反应参数并快速合成所需的分子库。新自动生成的数字代码已在各种活性药物成分的后期功能化方面进行了进一步测试。本文章由计算机程序翻译,如有差异,请以英文原文为准。
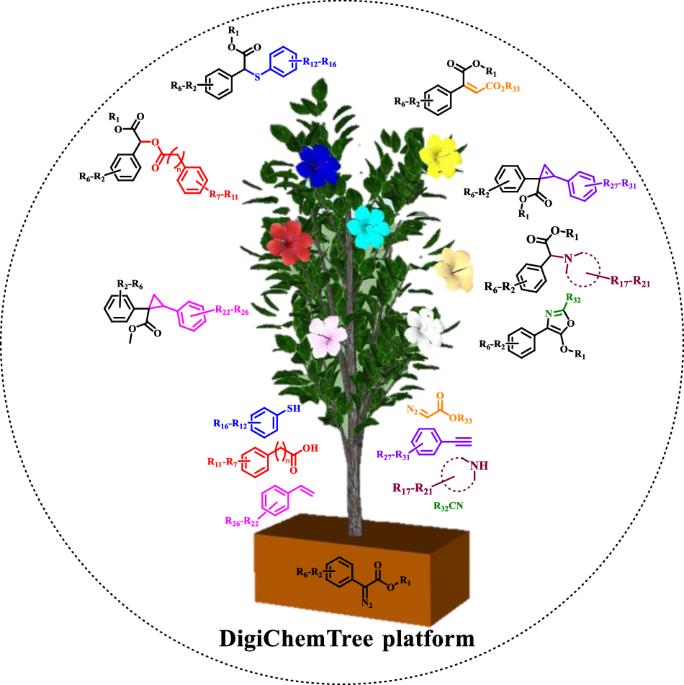
DigiChemTree enables programmable light-induced carbene generation for on demand chemical synthesis
The reproducibility of chemical reactions, when obtaining protocols from literature or databases, is highly challenging for academicians, industry professionals and even now for the machine learning process. To synthesize the organic molecule under the photochemical condition, several years for the reaction optimization, highly skilled manpower, long reaction time etc. are needed, resulting in non-affordability and slow down the research and development. Herein, we have introduced the DigiChemTree backed with the artificial intelligence to auto-optimize the photochemical reaction parameter and synthesizing the on demand library of the molecules in fast manner. Newly, auto-generated digital code was further tested for the late stage functionalization of the various active pharmaceutical ingredient. Light-induced reactions of diazo compounds have become crucial in organic synthesis and drug discovery, however, optimization of reaction conditions is still very time-consuming. Here, the authors develop a DigiChemTree platform using artificial intelligence to auto-optimize the photochemical reaction parameters and rapidly synthesize an on-demand library of molecules.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


