突变积累对线粒体功能和健康的累积效应。
IF 4.5
3区 生物学
Q2 CELL BIOLOGY
引用次数: 0
摘要
突变对线粒体的影响值得特别关注,因为这些细胞器对许多细胞功能起着至关重要的作用。本研究探讨了重复瓶颈对线粒体功能和适应性的影响。水蚤突变积累系(MA)在有铜和无铜条件下维持了 120 多代。突变积累品系繁殖后,将突变积累品系的水蚤在最适温度和高温条件下饲养两代,然后评估线粒体和表型特征。铜条件下的自发突变积累导致 MA 品系的成熟年龄推迟,繁殖力降低。与非突变积累(MA)对照相比,所有突变积累(MA)品系的线粒体呼吸都降低了 10%。在最适温度下,MA品系的MtDNA拷贝数比对照高,这表明存在补偿机制。在低铜条件下繁殖的三个 MA 株系的 mtDNA 拷贝数和适应性都很低,这表明突变可能影响了参与 mtDNA 复制或线粒体生物发生的基因。总之,我们的研究表明,突变积累对生活史特征、mtDNA拷贝数和线粒体呼吸都有影响。在高温条件下,一些表型效应被放大。MtDNA拷贝数似乎是一个重要的缓解因素,可使线粒体应对突变积累,但当突变积累达到一定程度后,线粒体就无法再进行补偿。本文章由计算机程序翻译,如有差异,请以英文原文为准。
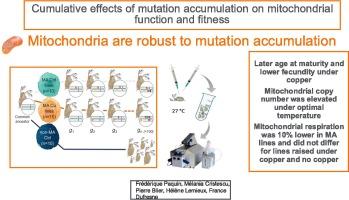
Cumulative effects of mutation accumulation on mitochondrial function and fitness
The impact of mutations on the mitochondria deserves specific interest due to the crucial role played by these organelles on numerous cellular functions. This study examines the effects of repeated bottlenecks on mitochondrial function and fitness. Daphnia pulex mutation accumulation lines (MA) lines were maintained for over 120 generations under copper and no copper conditions. Following the MA propagation, Daphnia from MA lines were raised under optimal and high temperatures for two generations before assessing mitochondrial and phenotypic traits. Spontaneous mutation accumulation under copper led to a later age at maturity and lowered fecundity in the MA lines. Mitochondrial respiration was found to be 10% lower in all mutation accumulation (MA) lines as compared to the non-MA control. MtDNA copy number was elevated in MA lines compared to the control under optimal temperature suggesting a compensatory mechanism. Three MA lines propagated under low copper had very low mtDNA copy number and fitness, suggesting mutations might have affected genes involved in mtDNA replication or mitochondrial biogenesis. Overall, our study suggests that mutation accumulation had an impact on life history traits, mtDNA copy number, and mitochondrial respiration. Some phenotypic effects were magnified under high temperatures. MtDNA copy number appears to be an important mitigation factor to allow mitochondria to cope with mutation accumulation up to a certain level beyond which it can no longer compensate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Mitochondrion
生物-细胞生物学
CiteScore
9.40
自引率
4.50%
发文量
86
审稿时长
13.6 weeks
期刊介绍:
Mitochondrion is a definitive, high profile, peer-reviewed international research journal. The scope of Mitochondrion is broad, reporting on basic science of mitochondria from all organisms and from basic research to pathology and clinical aspects of mitochondrial diseases. The journal welcomes original contributions from investigators working in diverse sub-disciplines such as evolution, biophysics, biochemistry, molecular and cell biology, genetics, pharmacology, toxicology, forensic science, programmed cell death, aging, cancer and clinical features of mitochondrial diseases.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


