Notch信号通过Hes1和HeyL调控中华绒螯蟹的肢体再生
IF 3.2
2区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
组织再生是动物补偿受损组织的一种有效策略,涉及各种类型的祖细胞。破译再生过程中调节这些祖细胞活性的信号网络对于理解不同物种再生能力的差异至关重要。在这项研究中,我们评估了节肢动物中华绒螯蟹肢体再生过程中 Notch 信号的表达谱和表型功能。Notch信号通路关键组分的表达在断肢后7天(7 DPA)时上调,随后在断肢后18天(18 DPA)时下降。为了评估Notch的作用,我们向自动切除区域注射了靶向Notch基因的dsRNA,并评估了再生效率。结果表明,阻断Notch信号导致再生缺陷,表现为伤口闭合和胚泡出现过程的延迟。此外,dsRNA敲除Notch信号后,Notch靶基因Hes1和HeyL的表达明显减少。Hes1的敲除特异性地损害了神经祖细胞标记的增殖和表达,而不影响成肌细胞。相反,HeyL的阻断抑制了活化的神经原祖细胞和肌原祖细胞的增殖和标记表达,同时上调了静止神经祖细胞的标记。这些研究结果表明,Notch信号通过激活下游效应物Hes1和HeyL,通过不同的机制调节神经发生和肌发生,在中华鳖的肢体再生过程中发挥了重要作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
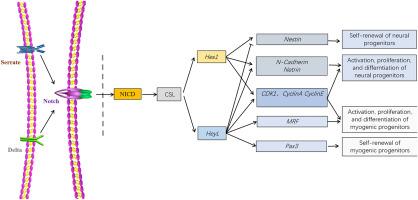
Notch signaling regulates limb regeneration through Hes1 and HeyL in the Chinese mitten crab
Tissue regeneration is an efficient strategy developed by animals to compensate for damaged tissues, involving various types of progenitor cells. Deciphering the signal network that modulates the activity of these progenitors during regeneration is crucial for understanding the differences in regenerative capacities across species. In this study, we evaluated the expression profile and phenotypic function of Notch signaling during limb regeneration in arthropod Chinese mitten crabs. The expression of key components of the Notch signaling pathway was upregulated at 7-day post-autotomy (7 DPA), and declined later at 18-day post-autotomy (18 DPA). To assess the role of Notch, we injected dsRNA targeting the Notch gene into the automized area and evaluated the regeneration efficiency. Our results indicated that blocking Notch signaling led to regenerative defects, manifested by delays in the wound closure and blastema emergence processes. Furthermore, the expression of Notch target genes, Hes1 and HeyL, was significantly reduced following Notch knockdown by dsRNA. Knockdown of Hes1 specifically impaired the proliferation and expression of neural progenitor cell markers, without affecting myogenic cells. In contrast, blockage of HeyL inhibited the proliferation and expression of markers in both activated neurogenic and myogenic progenitor cells, while up-regulating markers of quiescent neural progenitor cells. These findings suggest that Notch signaling plays an important role in limb regeneration of E. sinensis by activating downstream effectors Hes1 and HeyL, regulating neurogenesis and myogenesis through distinct mechanisms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.40
自引率
5.30%
发文量
105
审稿时长
40 days
期刊介绍:
This international journal publishes original contributions and mini-reviews in the fields of insect biochemistry and insect molecular biology. Main areas of interest are neurochemistry, hormone and pheromone biochemistry, enzymes and metabolism, hormone action and gene regulation, gene characterization and structure, pharmacology, immunology and cell and tissue culture. Papers on the biochemistry and molecular biology of other groups of arthropods are published if of general interest to the readership. Technique papers will be considered for publication if they significantly advance the field of insect biochemistry and molecular biology in the opinion of the Editors and Editorial Board.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


