阳离子交换膜特性在使用酸性溶解液电解 CO2 到 CO 中的作用
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
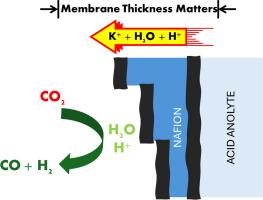
由于阳离子交换膜具有避免碳化和提高碳效率的潜力,因此被认为是零间隙二氧化碳电解的合适选择。然而,由于制氢量高,使用酸性溶液仍是一个问题。本研究调查了在含有 K2SO4 的酸性溶液的零间隙电池中,通过挤压或溶液浇铸工艺生产的不同厚度的 Nafion® 膜(111、112、115、211 和 212)。无论生产工艺如何,较薄的膜产生二氧化碳的法拉第效率(FECO)较高,在 50 mA cm-² 的条件下,FECO 达到 75% 左右。此外,在 30°C 和 50 mA cm-² 条件下电解 3 小时后,厚度相似(50.8 µm)但制造方法不同的膜会出现流场碳化现象。全电池和半电池配置下的线性扫描伏安法(LSV)显示,无论膜的厚度如何,所有采用的膜都具有相对于质子传输的极限扩散电流(iL)。相比之下,较厚的 Nafion® 115 膜的 iL 被抑制,这表明质子耗竭速度很快,在这种情况下,电极表面碱化主要是由水还原造成的。为解释氩气和二氧化碳馈入电池中的极限电流行为,进行了机理分析,结果表明二氧化碳还原有助于消耗膜提供的 H+,在负电位较低时提高局部 pH 值。总体而言,较薄的膜表现出较高的 FECO 值和 CO 能量效率(EE%CO)。然而,溶液浇铸膜更容易提供 K+,因此比挤压制备的膜性能更好。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The role of cation exchange membrane characteristics in CO2 electrolysis to CO using acid anolyte
Cation exchange membranes are considered a suitable option for zero-gap CO2 electrolysis due to their potential to avoid carbonation and improve carbon efficiency. However, the use of acidic anolytes remains an issue due to high hydrogen production. This study investigates Nafion® membranes (111, 112, 115, 211, and 212) with different thicknesses produced by extrusion or solution-cast processes in a zero-gap cell with an acidic anolyte containing K2SO4. Faradaic efficiencies for CO production (FECO) are higher with thinner membranes, regardless of the manufacturing process, reaching FECO around 75 % at 50 mA cm⁻². Additionally, membranes with similar thicknesses (∼50.8 µm) but produced in different ways displayed flow field carbonation after 3 h of electrolysis at 30 °C and 50 mA cm⁻². Linear sweep voltammetry (LSV) in full and half-cell configurations shows limiting diffusion current (iL) relative to proton transport for all the employed membranes, no matter the thickness. In contrast, the iL for Nafion® 115, the thicker membrane, is suppressed, indicating that proton depletion is fast and the electrode surface alkalinization primarily results from water reduction in this case. A mechanistic analysis was performed to explain the behavior of the limiting currents in the cell with Ar- and CO2-feed, indicating that CO2 reduction aids in the consumption of H+ provided by the membrane, increasing the local pH at less negative potentials. Overall, thinner membranes exhibited higher values of FECO and energy efficiency for CO (EE%CO). However, solution-cast membranes are more prone to provide K+, leading to better performance than those prepared by extrusion.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


