镍@金泡沫电极上葡萄糖对葡萄糖酸的选择性电氧化作用
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
Ni@Au 电极是通过金原子电化学置换商用泡沫镍中的镍原子而制备的。理化特性表明,金原子比率约为 6%,与电化学置换时间(1、2 和 3 分钟)无关,但沉积金层的结构存在差异。在 0.1 M NaOH 水溶液电解液中记录的循环伏安图的形状表明,金和镍的位点都是可接触的。在 0.1 M 葡萄糖存在下,所有 Ni@Au 电极都记录到了相同的氧化起始电位(与 RHE 相比约为 0.3 V),在达到的几何电流密度方面也具有相似的活性。在 Ni@Au 电极上对 0.1 M KOH 电解液中的 0.1 M 葡萄糖进行长期电解,电解槽电压分别为 0.575 V、0.675 V 和 0.775 V(相对于 RHE),结果表明在 5 小时内具有令人惊讶的出色稳定性,这是因为在与沉积金层接触的镍泡沫表面存在镍(OH)2 层。在 3 分钟沉积后获得的镍金电极上进行 5 小时电解后,转化率高达 60%,所有电极和 0.575 V 对 RHE 的较低电位对葡萄糖酸的选择性和远红外效率均为 100%。增加葡萄糖和 KOH 的初始浓度会降低转化率、选择性和法拉第效率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
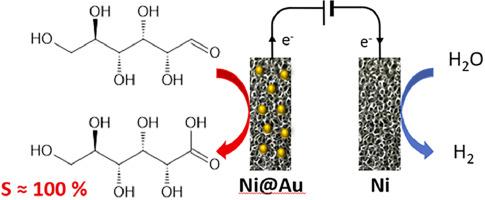
Selective electrooxidation of glucose towards gluconic acid on Ni@Au foam electrodes
Ni@Au electrodes are prepared by galvanic replacement of Ni atoms of a commercial Ni foam by Au atoms. The physicochemical characterizations indicate gold atomic ratios of ca. 6 %, independently on the galvanic replacement time (1, 2 and 3 min), but differences in the structure of the deposited gold layers. The shapes of the cyclic voltammograms recorded in a 0.1 M NaOH aqueous electrolyte indicate that both Au and Ni sites are accessible. In the presence of 0.1 M glucose, the same oxidation onset potential of ca. 0.3 V vs RHE and a comparable activity in terms of achieved geometric current densities were recorded for all the Ni@Au electrodes. The long term electrolyses of 0.1 M glucose in 0.1 M aqueous KOH electrolyte on the Ni@Au electrodes performed at cell voltages corresponding to anode potentials of 0.575 V, 0.675 V and 0.775 V vs RHE show a surprising excellent stability over 5 h, which is explained by the presence of a Ni(OH)2 layer on the surface of the Ni foam in contact with the deposited gold layers. Conversions up to 60 % are obtained after 5 h electrolyses with the Ni-Au electrode obtained after 3 min deposition, with 100 % selectivity and faradaic efficiency towards gluconic acid for all the electrodes and for the lower potential of 0.575 V vs RHE. Increasing the glucose and KOH initial concentrations decreases the conversion rate, selectivity and faradaic efficiency.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


