在结构引导下,从 AlphaFold 蛋白结构数据库中发现并合理设计新型聚对苯二甲酸乙二醇酯水解酶
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
酶降解为解决塑料污染,尤其是聚对苯二甲酸乙二酯(PET)污染提供了一种前景广阔的生态友好型解决方案。目前的工作重点是从微生物和元基因组中筛选 PET 降解酶,并通过有限的模板集获得优质候选酶。PET 废料生物炼制需要更高效的 PET 水解酶。在此,我们利用结构引导的生物信息学工作流程,通过筛选 AlphaFold 蛋白结构数据库,从小孢子菌 HM5-17 中发现了一种新型 PET 加氢酶 LSPET4。LSPET4 具有独特的碳水化合物结合模块(CBM)和独特的线性底物结合构象。与之前报道的最有效的 CBM3 相比,LSPET4 中固有的 CBM 在 PET 表面上表现出更优越的结合能力,并增强了 PET 的水解性能。通过合理的蛋白质工程学研究,我们开发出了 LSPET4M6(D130P、N127F、Y96F、Q209E、A238K、D241S),与野生型相比,该变体的活性提高了 38.79 倍,与已报道的源自 IsPETase 的最有效 PET 水解酶 FAST-PETase 在 45℃ 下的活性相当。该变体在降解各种商用 PET 材料(包括食品工业中使用的 PET 食品封口膜、PET 草莓盒和 PET 番茄盒)方面也表现出了有效性。这项研究不仅为蛋白质工程工作提供了一个新模板,以创造用于 PET 回收的高效生物催化剂,而且还提供了一种有效的酶发现方法,从 AlphaFold 蛋白结构数据库中发现感兴趣的酶。本文章由计算机程序翻译,如有差异,请以英文原文为准。
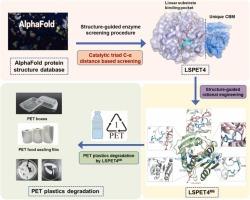
Structure-guided discovery and rational design of a new poly(ethylene terephthalate) hydrolase from AlphaFold protein structure database
Enzymatic degradation offers a promising eco-friendly solution to plastic pollution, especially for polyethylene terephthalate (PET). Current efforts have focused on screening PET-degrading enzymes from microbial and metagenomic sources and obtaining superior candidates with a limited set of templates. More efficient PET hydrolases are required for PET-waste biorefinery. Here, using a structure-guided bioinformatic workflow, we identified a novel PET hydrolase, LSPET4, from Micromonospora sp. HM5-17, by screening the AlphaFold protein structure database. LSPET4 features a unique carbohydrate-binding module (CBM) and a distinctive linear substrate binding conformation. The intrinsic CBM in LSPET4 exhibited superior binding ability on PET surfaces and enhanced PET hydrolysis performance compared to the previously reported most effective CBM3. Through rational protein engineering focused on stabilizing and modifying the linear substrate binding conformation, we developed LSPET4M6 (D130P, N127F, Y96F, Q209E, A238K, D241S), a variant that achieved a 38.79-fold improvement in activity compared to the wild type, and was comparable to the reported most effective PET hydrolase derived from IsPETase, FAST-PETase at 45℃. This variant also demonstrated effectiveness in degrading various commercial PET materials, including PET food sealing films, PET strawberry boxes, and PET tomato boxes used in the food industry. This study not only provides a new template for protein engineering endeavors to create efficient biocatalysts for PET recycling but also offers an effective enzyme discovery approach to uncover enzymes of interest from the AlphaFold protein structure database.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


