解密金属硫化物电极的多电子氧化还原化学,实现先进的水性铜离子存储
IF 11.2
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
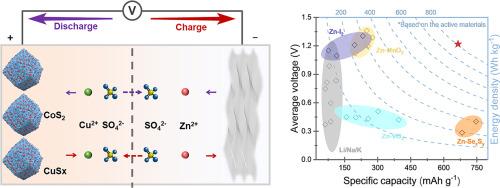
中性水溶液金属电池具有成本效益高、不易燃等特点,在大规模储能方面具有巨大潜力,但其实际应用却受到阴极材料比容量有限(小于 500 mAh g-1)的阻碍。本文基于水性铜离子体系开发了容量导向型 CoS2 和速率优化型 Co9S8 阴极。通过一系列原位测试和密度泛函理论计算,重点研究了 Co9S8→Cu7S4→Cu9S5/Cu1.8S 和 CoS2→Cu7S4→Cu2S 的电荷存储机理,这些机理分别与 Cu2+/Cu+‖Co2+/Co 和 Cu2+/Cu+‖S22-/S2- 的氧化还原反应有关。电化学结果表明,CoS2 在 1 A g-1 循环 400 次后可显示出 619 mAh g-1 的超强容量,而 Co9S8 在 10 A g-1 循环时可保持 497 mAh g-1 的出色速率性能(与 1 A g-1 循环时的 521 mAh g-1 相比,保持率为 95%)。作为概念验证,先进的 CoS2//Zn 混合水电池的工作电压为 1.20 V,比能量为 663 Wh kgcathode-1。这项研究为在高能水性金属电池中开发硫化物阴极提供了另一个方向。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Deciphering the multi-electron redox chemistry of metal-sulfide electrode toward advanced aqueous Cu ion storage
While neutral aqueous metal batteries, featuring cost-effectiveness and non-flammability, hold significant potential for large-scale energy storage, their practical application is hampered by the limited specific capacity of cathode materials (less than 500 mAh g−1). Herein, capacity-oriented CoS2 and rate-optimized Co9S8 cathodes are developed based on the aqueous copper ion system. The charge-storage mechanism is systematically investigated through a series of ex-situ tests and density functional theory calculations, focusing on the reversible transitions of Co9S8→Cu7S4→Cu9S5/Cu1.8S and CoS2→Cu7S4→Cu2S, which are associated with the redox reactions of Cu2+/Cu+‖Co2+/Co and Cu2+/Cu+‖S22−/S2−, respectively. The electrochemical results show that CoS2 can exhibit a superior capacity of 619 mAh g−1 at 1 A g−1 after 400 cycles, while Co9S8 maintains an outstanding rate performance of 497 mAh g−1 at 10 A g−1 (the retention rate is 95% compared to 521 mAh g−1 at 1 A g−1). As a proof of concept, an advanced CoS2//Zn hybrid aqueous battery demonstrates a working voltage of 1.20 V and a specific energy of 663 Wh kgcathode−1. This work provides an alternative direction for developing sulfide cathodes in energetic aqueous metal batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Materials Science & Technology
工程技术-材料科学:综合
CiteScore
20.00
自引率
11.00%
发文量
995
审稿时长
13 days
期刊介绍:
Journal of Materials Science & Technology strives to promote global collaboration in the field of materials science and technology. It primarily publishes original research papers, invited review articles, letters, research notes, and summaries of scientific achievements. The journal covers a wide range of materials science and technology topics, including metallic materials, inorganic nonmetallic materials, and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


