镍催化的烯醇衍生物还原烯化反应:构建烯烃的多功能工具
IF 17.7
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
酮到烯的转化在有机合成中至关重要,而涉及烯醇衍生物的过渡金属催化交叉偶联反应已成为实现这一目标的有力工具。在亲核-亲电反应取得重大进展的同时,镍催化的还原烯化反应的最新进展也引起了越来越多的关注。这些方法适用于多种官能团,如醛、酮、酰胺、醇、炔、杂环和有机锡化合物,为获得结构多样的烯烃提供了一种有效的策略。本《账户》主要介绍了我们实验室在这一不断发展的领域中做出的贡献,同时也感谢其他研究人员做出的重要贡献。本文章由计算机程序翻译,如有差异,请以英文原文为准。
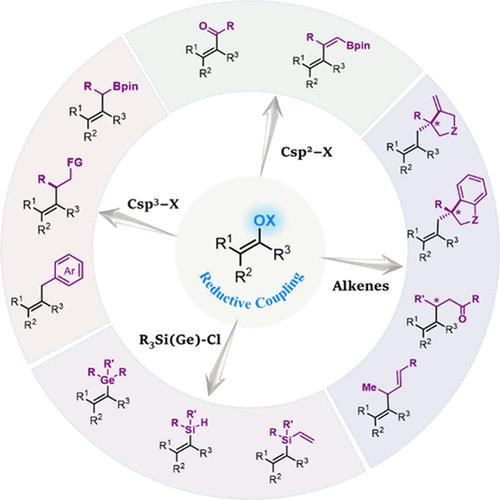
Nickel-Catalyzed Reductive Alkenylation of Enol Derivatives: A Versatile Tool for Alkene Construction
Ketone-to-alkene transformations are essential in organic synthesis, and transition-metal-catalyzed cross-coupling reactions involving enol derivatives have become powerful tools to achieve this goal. While substantial progress has been made in nucleophile–electrophile reactions, recent developments in nickel-catalyzed reductive alkenylation reactions have garnered increasing attention. These methods accommodate a broad range of functional groups such as aldehyde, ketone, amide, alcohol, alkyne, heterocycles, and organotin compounds, providing an efficient strategy to access structurally diverse alkenes. This Account primarily highlights the contributions from our laboratory to this growing field while also acknowledging key contributions from other researchers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Accounts of Chemical Research
化学-化学综合
CiteScore
31.40
自引率
1.10%
发文量
312
审稿时长
2 months
期刊介绍:
Accounts of Chemical Research presents short, concise and critical articles offering easy-to-read overviews of basic research and applications in all areas of chemistry and biochemistry. These short reviews focus on research from the author’s own laboratory and are designed to teach the reader about a research project. In addition, Accounts of Chemical Research publishes commentaries that give an informed opinion on a current research problem. Special Issues online are devoted to a single topic of unusual activity and significance.
Accounts of Chemical Research replaces the traditional article abstract with an article "Conspectus." These entries synopsize the research affording the reader a closer look at the content and significance of an article. Through this provision of a more detailed description of the article contents, the Conspectus enhances the article's discoverability by search engines and the exposure for the research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


