新型查耳酮半咔唑酮(S,Se)及其偶氮唑衍生物的合成及其对南美锥虫病的生物学评价
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
南美锥虫病是由真核寄生虫克鲁斯锥虫引起的。目前的治疗方法疗效有限,而硒基化合物作为新疗法的候选化合物大有可为,其疗效已超过其生物异构体--硫。我们利用同位取代技术设计了新的硫代氨基甲酸酯、噻唑、硒代氨基甲酸酯和硒唑。我们合成了 57 种新的查耳酮化合物,并对它们进行了针对 T. cruzi、C2C12 细胞和 cruzain(该寄生虫的主要靶标)的评估。此外,我们还测试了人类 cathepsin L 的选择性。根据其对细胞内母细胞的活性(EC50 < 1 μM,SI > 10)和对克鲁泽因的活性(IC50 < 100 nM,SI > 5.55),选出了三种化合物,它们与已获批准的药物苯并咪唑和成熟的克鲁泽因抑制剂 K777 相比效果更佳。硒化合物显示出更强的活性,而硒唑类化合物则显示出与硒相关的毒性有所降低。化合物 4-甲基-2-(2-(1-(3-硝基苯基)亚乙基)肼基)-1,3-硒唑(Se2h)成为有希望的候选化合物,并研究了它与克鲁扎因的结合。进行了药代动力学评估,结果表明其对随后的体内试验具有有利影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
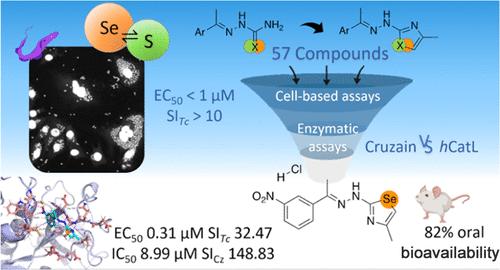
Synthesis and Biological Evaluation of New Chalcogen Semicarbazone (S, Se) and Their Azole Derivatives against Chagas Disease
Chagas disease is caused by the eukaryote parasite Trypanosoma cruzi. Current treatment exhibits limited efficacy and selenium-based compounds emerged as promising candidates for new therapies which is surpassing its bioisoster, sulfur. We designed new thiosemicarbazones, thiazoles, selenosemicarbazones and selenazoles, using isosteric substitution. We synthesized 57 new chalcogen compounds which were evaluated against T. cruzi, C2C12 cells and cruzain, the main target of this parasite. Additionally, human cathepsin L, was tested for selectivity. Three compounds were selected, based on their activity against the intracellular amastigotes (EC50 < 1 μM, SI > 10) and cruzain (IC50 < 100 nM, SI > 5.55) which compared favorably with the approved drug, Benznidazole, and the well-established cruzain inhibitor K777. Seleno-compounds demonstrated enhanced activity and selenazoles showed a decrease in selenium-associated toxicity. Compound 4-methyl-2-(2-(1-(3-nitrophenyl)ethylidene)hydrazineyl)-1,3-selenazole (Se2h) emerged as a promising candidate, and its binding to cruzain was investigated. Pharmacokinetic assessment was conducted, showing a favorable profile for subsequent in vivo assays.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


