琼脂降解糖苷酶中不寻常的 His/Asp 二联体起催化作用
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
红藻细胞壁中的琼脂糖图案由交替存在的 d-半乳糖(G)和 α-3,6-脱水-l-半乳糖(LA)单元组成。117 家族(GH117)的糖苷水解酶可裂解末端的 α-1,3-糖苷键,释放出 LA 单元。结构研究表明,这些酶使用非常规催化机制,涉及组氨酸(His302)作为一般酸,而不是大多数糖苷酶中的羧基残基。通过量子力学/分子力学元动力学,我们研究了 Phocaeicola plebeius GH117 的反应机制,证实了 His302 的催化作用。该残基与邻近的天冬氨酸残基(Asp320)共享一个质子,形成 His/Asp 二元组。我们的研究还发现,尽管位于-1位点(LA)的糖单元可以采用两种构象,即 4C1 和 1,4B,但只有后者才具有催化作用,从而确定了 1,4B → [4E]‡ → 1,4B (→ 4C1) 的构象行程。这种机制可能适用于活性位点中含有 His/Asp 二联体的类似酶,如 GH3 β-N-乙酰葡糖苷酶和 GH156 丝氨酸酶。这些见解加深了我们对糖苷酶催化策略的理解,可为更有效地加工海藻的酶工程提供信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
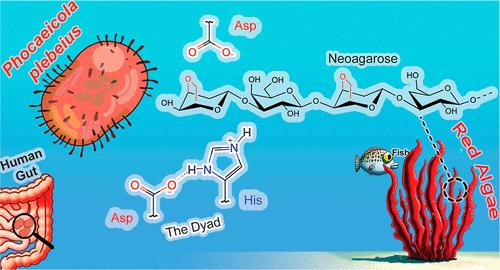
An Unusual His/Asp Dyad Operates Catalysis in Agar-Degrading Glycosidases
Agarose motifs, found in agars present in the cell walls of red algae, consist of alternating units of d-galactose (G) and α-3,6-anhydro-l-galactose (LA). Glycoside hydrolases from family 117 (GH117) cleave the terminal α-1,3-glycosidic bonds, releasing LA units. Structural studies have suggested that these enzymes use unconventional catalytic machinery, involving a histidine (His302) as a general acid rather than a carboxylic residue as in most glycosidases. By means of quantum mechanics/molecular mechanics metadynamics, we investigated the reaction mechanism of Phocaeicola plebeius GH117, confirming the catalytic role of His302. This residue shares a proton with a neighbor aspartate residue (Asp320), forming a His/Asp dyad. Our study also reveals that, even though the sugar unit at the –1 subsite (LA) can adopt two conformations, 4C1 and 1,4B, only the latter is catalytically competent, defining a 1,4B → [4E]‡ → 1,4B (→ 4C1) conformational itinerary. This mechanism may be applicable to similar enzymes with a His/Asp dyad in their active sites, such as GH3 β-N-acetylglucosaminidase and GH156 sialidase. These insights enhance our understanding of glycosidase catalytic strategies and could inform the engineering of enzymes for the more efficient processing of seaweed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


