锆-钌异质核催化胺-硼烷脱氢过程中按需进行的金属对金属电子捐献
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在涉及活化具有强键的小分子的催化过程中,双核金属-金属协同效应非常重要。在此,我们报告了密度泛函理论计算的结果,这些计算用于确定 Zr-Ru 异核复合物催化胺硼烷脱氢过程中的催化机理和金属-金属协同效应。这些计算显示,在催化键活化过程中,步骤主要发生在 Zr 中心,而 Ru 金属则扮演着类似配体的按需电子捐赠伙伴的角色。我们还利用计算确定了双核 Zr-Ru 复合物与单核 Zr 和 Ru 复合物之间的机理和反应性差异。本文章由计算机程序翻译,如有差异,请以英文原文为准。
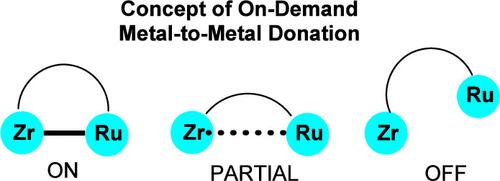
On-Demand Metal-to-Metal Electron Donation during Zr–Ru Heterodinuclear-Catalyzed Amine–Borane Dehydrogenation
Dinuclear metal–metal cooperative effects are important in catalysis involving the activation of small molecules with strong bonds. Here, we report density functional theory calculations used to determine the catalytic mechanism and metal–metal cooperative effects during amine–borane dehydrogenation catalyzed by a Zr–Ru heterodinuclear complex. These calculations revealed that, during catalysis bond activation, steps occur mainly at the Zr center and the Ru metal plays a role as a ligand-like on-demand electron donation partner. We also used calculations to determine the mechanistic and reactivity differences between the dinuclear Zr–Ru complex and mononuclear Zr and Ru complexes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


