库普弗细胞逆向迁移到肝窦可减轻新生儿败血症和脑膜炎的病情
IF 17.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
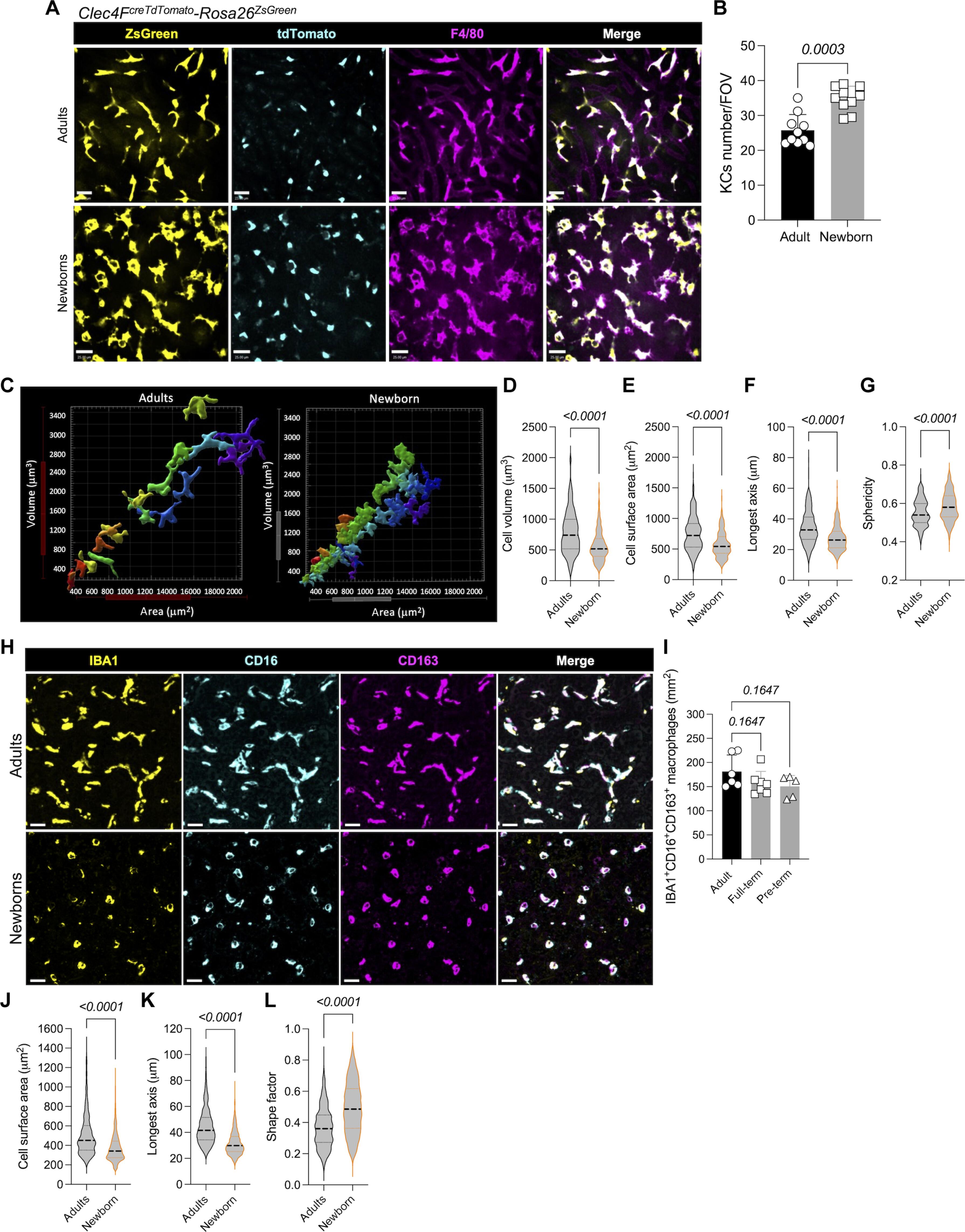
在成人体内,肝脏驻留的巨噬细胞或库普弗细胞(Kupffer cells,KCs)驻留在静脉窦中,通过捕捉快速流动的微生物来消毒循环血液。我们对出生 1 天的小鼠进行了定量眼内成像,并结合转录组学、遗传操作和体内感染试验,以研究新生儿对血流感染的易感性。出生 1 天的 KC 在体外捕捉大肠杆菌的能力更强,而我们发现产后 1 周是一个关键窗口期,此时 KC 获取血液的机会有限,必须从肝实质转移到肝窦。KC 的迁移与微生物群无关,但依赖于巨噬细胞迁移抑制因子、其受体 CD74 和粘附分子 CD44。根据我们的研究结果,我们提出了一个原代巨噬细胞通过从肝实质逆向迁移过程播种到肝窦的模型。这些结果还说明了开发新生小鼠模型对了解新生儿免疫和疾病的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Kupffer cell reverse migration into the liver sinusoids mitigates neonatal sepsis and meningitis
In adults, liver-resident macrophages, or Kupffer cells (KCs), reside in the sinusoids and sterilize circulating blood by capturing rapidly flowing microbes. We developed quantitative intravital imaging of 1-day-old mice combined with transcriptomics, genetic manipulation, and in vivo infection assays to interrogate increased susceptibility of newborns to bloodstream infections. Whereas 1-day-old KCs were better at catching Escherichia coli in vitro, we uncovered a critical 1-week window postpartum when KCs have limited access to blood and must translocate from liver parenchyma into the sinusoids. KC migration was independent of the microbiome but depended on macrophage migration inhibitory factor, its receptor CD74, and the adhesion molecule CD44. On the basis of our findings, we propose a model of progenitor macrophage seeding of the liver sinusoids via a reverse transmigration process from liver parenchyma. These results also illustrate the importance of developing newborn mouse models to understand newborn immunity and disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Immunology
Immunology and Microbiology-Immunology
CiteScore
32.90
自引率
2.00%
发文量
183
期刊介绍:
Science Immunology is a peer-reviewed journal that publishes original research articles in the field of immunology. The journal encourages the submission of research findings from all areas of immunology, including studies on innate and adaptive immunity, immune cell development and differentiation, immunogenomics, systems immunology, structural immunology, antigen presentation, immunometabolism, and mucosal immunology. Additionally, the journal covers research on immune contributions to health and disease, such as host defense, inflammation, cancer immunology, autoimmunity, allergy, transplantation, and immunodeficiency. Science Immunology maintains the same high-quality standard as other journals in the Science family and aims to facilitate understanding of the immune system by showcasing innovative advances in immunology research from all organisms and model systems, including humans.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


