突变体 BCL11B-N440K 蛋白在 T 淋巴细胞和神经元发育过程中干扰 BCL11A 的功能
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
对小鼠的遗传研究表明,锌指转录因子 BCL11B 在调节早期 T 细胞发育和神经发生方面起着至关重要的作用。从一名患有 T 细胞缺乏症和神经系统疾病的患者体内分离出了一种新的杂合性错义 BCL11B 变体 BCL11BN441K。在这里,我们发现携带相应 Bcl11bN440K 突变的小鼠胸腺中出现了类似于天然杀伤细胞(NK)/第一类先天性淋巴细胞(ILC1)的 NKp46+ 细胞,而新皮质中的 TBR1+ 神经元则减少了。因此,突变体BCL11B-N440K蛋白在异源二聚化时会干扰BCL11A的功能。从机理上讲,Bcl11b-N440K 突变抑制了胸腺细胞中 BCL11B 与 T 细胞因子 1(TCF1)的相互作用,导致对 TCF1 活性的拮抗作用减弱,从而支持了 NK/ILC1 样细胞的分化。总之,我们的研究结果为我们揭示了 BCL11A 在抑制非 T 淋巴细胞发育潜能方面的功能,并揭示了 BCL11B-N440K 干扰 BCL11 家族伙伴蛋白的致病机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
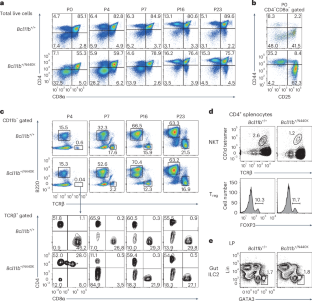
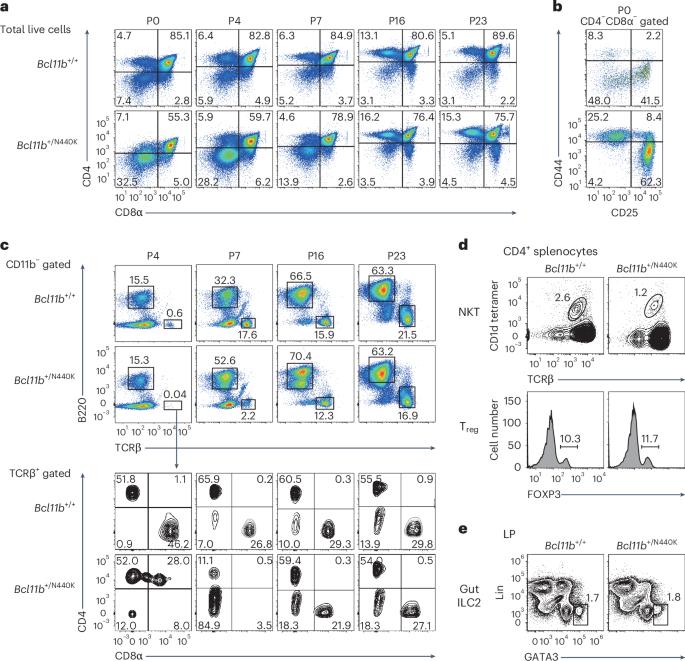
A mutant BCL11B-N440K protein interferes with BCL11A function during T lymphocyte and neuronal development
Genetic studies in mice have shown that the zinc finger transcription factor BCL11B has an essential role in regulating early T cell development and neurogenesis. A de novo heterozygous missense BCL11B variant, BCL11BN441K, was isolated from a patient with T cell deficiency and neurological disorders. Here, we show that mice harboring the corresponding Bcl11bN440K mutation show the emergence of natural killer (NK)/group 1 innate lymphoid cell (ILC1)-like NKp46+ cells in the thymus and reduction in TBR1+ neurons in the neocortex, which are observed with loss of Bcl11a but not Bcl11b. Thus, the mutant BCL11B-N440K protein interferes with BCL11A function upon heterodimerization. Mechanistically, the Bcl11bN440K mutation dampens the interaction of BCL11B with T cell factor 1 (TCF1) in thymocytes, resulting in weakened antagonism against TCF1 activity that supports the differentiation of NK/ILC1-like cells. Collectively, our results shed new light on the function of BCL11A in suppressing non-T lymphoid developmental potential and uncover the pathogenic mechanism by which BCL11B-N440K interferes with partner BCL11 family proteins. Taniuchi and colleagues describe the generation and phenotype of a mouse model carrying a defective Bcl11b allele encoding a mutant protein corresponding to BCL11B-N441K previously associated with human disease. They further characterize the molecular interactions between the mutant BCL11B protein and other transcription factors involved in early thymocyte development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


