高血糖引发的脂质过氧化会破坏 STAT4 的稳定性并损害 2 型糖尿病患者的 Th1 抗病毒反应
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
2型糖尿病(T2D)患者更容易受到严重的呼吸道病毒感染,但其潜在机制仍然难以捉摸。在这里,我们发现 T2D 患者和冠状病毒病 2019(COVID-19)感染者以及感染流感的 T2D 小鼠表现出 T 辅助细胞 1(Th1)反应缺陷,而 T 辅助细胞 1 是抗病毒免疫的重要组成部分。这种缺陷源于高血糖导致的 CD4+ T 细胞内在代谢紊乱。从机理上讲,高血糖会引发线粒体功能障碍和脂肪酸合成过多,导致氧化应激升高和 CD4+ T 细胞内脂质异常积累。这些异常会促进脂质过氧化(LPO),从而促使信号转导和激活转录 4(STAT4)发生羰基化,STAT4 是决定 Th1 线型的关键因子。羰基化的 STAT4 会迅速降解,导致 T-bet 诱导减少和 Th1 分化减弱。LPO 清除剂能改善血糖控制不佳的 T2D 患者的 Th1 缺陷,并能恢复 T2D 小鼠的病毒控制。因此,高血糖-LPO-STAT4轴是T2D宿主Th1活性降低的基础,对控制T2D相关病毒并发症具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
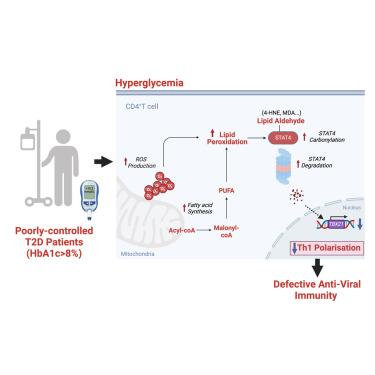
Hyperglycemia-triggered lipid peroxidation destabilizes STAT4 and impairs anti-viral Th1 responses in type 2 diabetes
Patients with type 2 diabetes (T2D) are more susceptible to severe respiratory viral infections, but the underlying mechanisms remain elusive. Here, we show that patients with T2D and coronavirus disease 2019 (COVID-19) infections, and influenza-infected T2D mice, exhibit defective T helper 1 (Th1) responses, which are an essential component of anti-viral immunity. This defect stems from intrinsic metabolic perturbations in CD4+ T cells driven by hyperglycemia. Mechanistically, hyperglycemia triggers mitochondrial dysfunction and excessive fatty acid synthesis, leading to elevated oxidative stress and aberrant lipid accumulation within CD4+ T cells. These abnormalities promote lipid peroxidation (LPO), which drives carbonylation of signal transducer and activator of transcription 4 (STAT4), a crucial Th1-lineage-determining factor. Carbonylated STAT4 undergoes rapid degradation, causing reduced T-bet induction and diminished Th1 differentiation. LPO scavenger ameliorates Th1 defects in patients with T2D who have poor glycemic control and restores viral control in T2D mice. Thus, this hyperglycemia-LPO-STAT4 axis underpins reduced Th1 activity in T2D hosts, with important implications for managing T2D-related viral complications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


