miR160触发的移动转录轴控制拟南芥根干细胞龛的维持和再生
IF 10.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
在多细胞生物体中,细胞之间的交流对其命运的决定至关重要。在植物中,静止中心(QC)向邻近的干细胞发出信号,使它们保持未分化状态。然而,人们对周围干细胞如何向QC发出信号仍知之甚少。在这里,我们发现,在拟南芥根部,microRNA160(miR160)从支柱干细胞(SSCs)移动到QC,并在QC降解ARF10和ARF17这两个辅助因子的mRNA。这种降解解除了对BRAVO的直接转录抑制,维持了QC的静止状态。我们进一步发现,DNA损伤诱导的SSC死亡和受限的交配运输阻断了miR160的移动,从而降低了BRAVO和WOX5的表达,导致QC分裂,在根再生过程中补充受损干细胞。我们的研究结果表明,由移动的miR160启动的转录轴调节QC和干细胞的行为,加深了我们对干细胞与其周围细胞环境之间交流的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
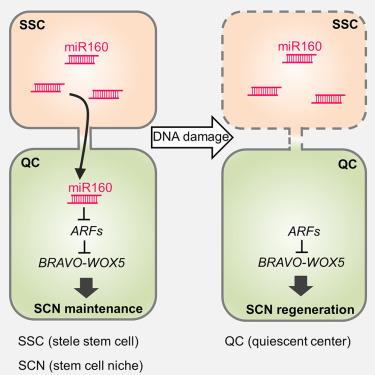
A mobile miR160-triggered transcriptional axis controls root stem cell niche maintenance and regeneration in Arabidopsis
In multicellular organisms, communication between cells is vital for their fate determination. In plants, the quiescent center (QC) signals to adjacent stem cells to maintain them undifferentiated. However, how surrounding stem cells instruct the QC remains poorly understood. Here, we show that in the Arabidopsis root, microRNA160 (miR160) moves from stele stem cells (SSCs) to the QC, where it degrades the mRNAs of two auxin response factors, ARF10 and ARF17. This degradation relieves BRAVO from direct transcriptional repression, maintaining QC quiescence. We further identify that blocking miR160 movement due to DNA damage-induced SSC death and restricted symplastic transport reduces BRAVO and WOX5 expression, leading to QC division to replenish damaged stem cells during root regeneration. Together, our results demonstrate that a transcriptional axis initiated by mobile miR160 regulates the QC and stem cell behavior, advancing our understanding of the communication between stem cells and their surrounding cellular environment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


