Tau 种子催化 GFP tau 生物传感器细胞形成纤维状结构
IF 4.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
在阿尔茨海默病(AD)和数十种牛头蛋白病中会出现牛头蛋白的纤维状聚集。纤维通过朊病毒样播种催化聚集,这也是疾病进展的部分原因。重组和脑源性 tau 纤维的播种是通过生物传感器细胞来测量的,这些细胞表达易聚集的 tau 突变体,并与荧光报告蛋白融合。播种会导致点状表型,这一点已经得到证实,但缺乏证据表明生物传感器细胞中的荧光 tau 融合蛋白会组装成纤维状结构。我们研究了播种对生物传感器细胞形成纤维的影响。被催化的荧光点状细胞表型以不同的稳定性持续存在。带有点状表型的播种细胞会产生 sarkosyl 不溶性纤维,而非播种细胞则不会。细胞纯化纤维的免疫组织电镜显示,GFP 定位于对蛋白水解敏感的 tau 纤维的模糊外衣上。这些数据提供了令人信服的证据,表明荧光点是朊病毒样播种产生的 tau 纤维聚集体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
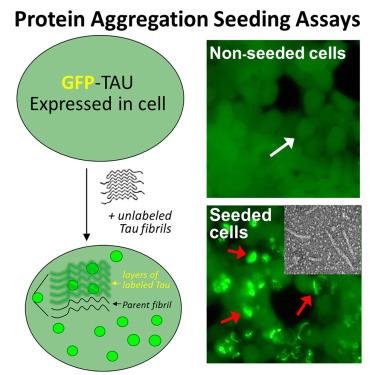
Tau seeds catalyze fibril-type structures from GFP tau biosensor cells
Fibril-type aggregates of tau occur in Alzheimer’s disease (AD) and dozens of tauopathies. Fibrils catalyze aggregation by prion-like seeding, which in part underlies disease progression. Seeding by recombinant and brain-derived tau fibrils is measured using biosensor cells that express aggregation-prone tau mutants fused with fluorescent reporter proteins. Seeding results in a punctated phenotype that is well established, but evidence that fluorescent tau fusion proteins from biosensor cells assemble into fibril-type structures is lacking. We investigated the effects of seeding on fibril formation by biosensor cells. Fluorescent punctated cell phenotypes that were catalyzed persisted with varying stabilities. Seeded cells bearing punctated phenotypes yielded sarkosyl-insoluble fibrils, although non-seeded cells did not. ImmunoEM of cell-purified fibrils shows that GFP localizes to the proteolytically sensitive fuzzy coat of tau fibrils. The presented data offer compelling evidence that fluorescent puncta are fibril-type aggregates of tau that result from prion-like seeding.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


