对 EGF20-27 区域的结构和功能研究揭示了人类 Notch 受体对于最佳激活的新特征
IF 4.3
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
Notch受体由Delta/Serrate/Lag-2(DSL)配体家族激活。尽管由配体结合区(LBR)和负调控区(NRR)组成的 Notch/配体复合物的结构已被解决,但细胞外信号复合物的组织结构尚不清楚。在这里,我们研究了人类 Notch-1 表皮生长因子样(EGF)20-27 区域,该区域位于配体结合区(LBR)和负调控区(NRR)之间,并包含与果蝇独特表型相关的 Abruptex(Ax)区域。我们利用晶体学、核磁共振和小角 X 射线散射(SAXS)进行了分析,结果表明 EGF20-27 具有刚性、拉长的组织结构,EGF20-21 的连接具有依赖 Ca2+ 的灵活性。在功能测试中,含有 Ax 取代的 Notch-1 变体会降低配体依赖性转激活。当表达顺式-JAG1 时,WT 和结合 Ca2+ 的 Ax 变体之间的 Notch 活性差异没有单独在反式激活实验中看到的那么明显,这与顺式抑制的破坏是一致的。这些数据表明了Ca2+稳定结构的重要性,并表明顺式和反式相互作用的平衡解释了果蝇Ax突变的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
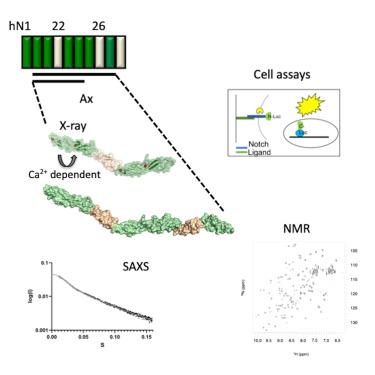
Structural and functional studies of the EGF20-27 region reveal new features of the human Notch receptor important for optimal activation
The Notch receptor is activated by the Delta/Serrate/Lag-2 (DSL) family of ligands. The organization of the extracellular signaling complex is unknown, although structures of Notch/ligand complexes comprising the ligand-binding region (LBR), and negative regulatory region (NRR) region, have been solved. Here, we investigate the human Notch-1 epidermal growth factor-like (EGF) 20-27 region, located between the LBR and NRR, and incorporating the Abruptex (Ax) region, associated with distinctive Drosophila phenotypes. Our analyses, using crystallography, NMR and small angle X-ray scattering (SAXS), support a rigid, elongated organization for EGF20-27 with the EGF20-21 linkage showing Ca2+-dependent flexibility. In functional assays, Notch-1 variants containing Ax substitutions result in reduced ligand-dependent trans-activation. When cis-JAG1 was expressed, Notch activity differences between WT and Ca2+-binding Ax variants were less marked than seen in the trans-activation assays alone, consistent with disruption of cis-inhibition. These data indicate the importance of Ca2+-stabilized structure and suggest the balance of cis- and trans-interactions explains the effects of Drosophila Ax mutations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


