致病性线粒体 DNA 变异抑制黑色素瘤转移
IF 11.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
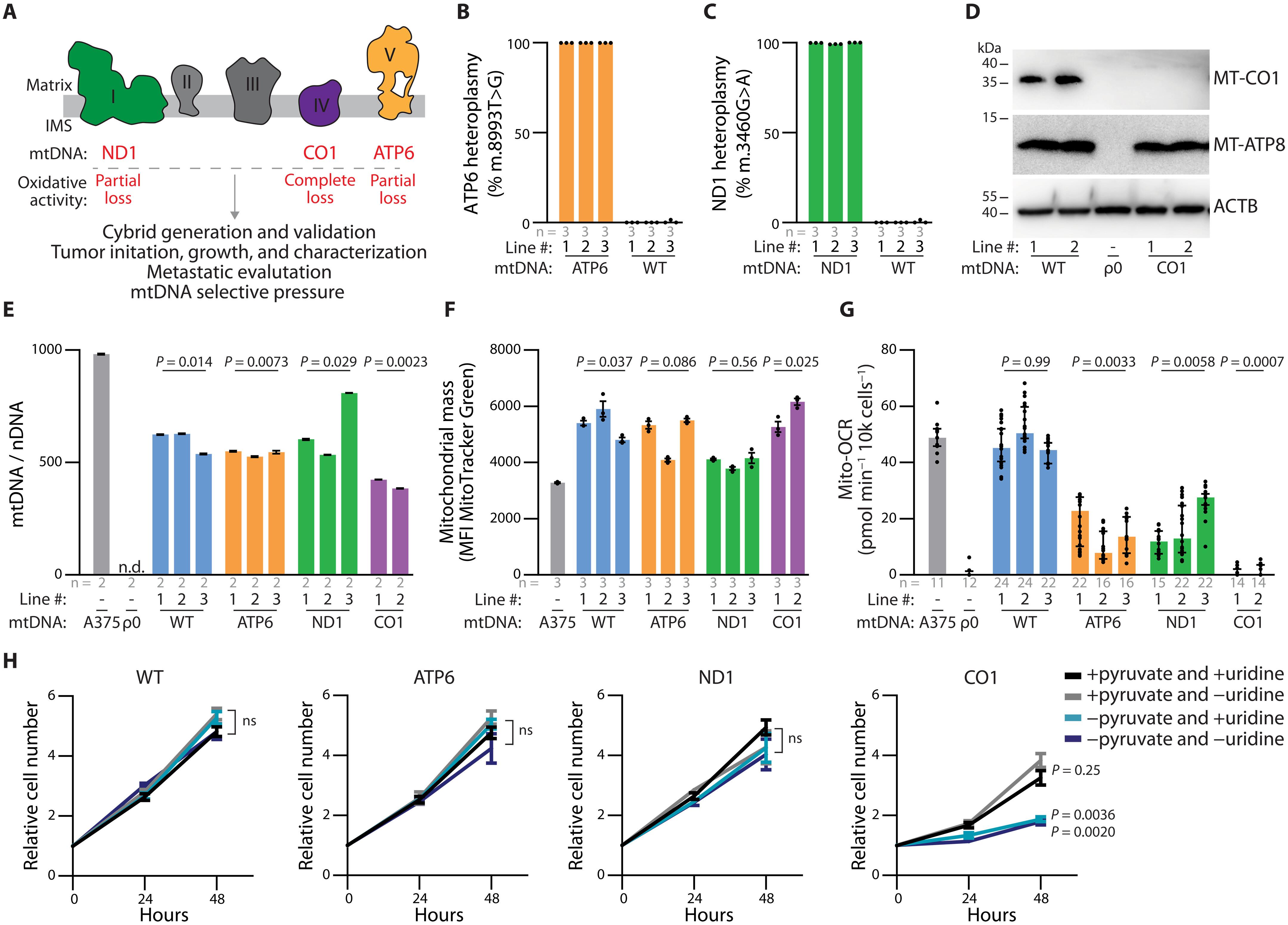
线粒体 DNA(mtDNA)突变在癌症中很常见,但它们在癌症进展中的确切作用仍存在争议。为了从功能上评估 mtDNA 变异对肿瘤生长和转移的影响,我们开发了一种增强型细胞质杂交(细胞杂交)生成方案,并建立了具有野生型 mtDNA 或部分或完全丧失线粒体氧化功能的致病性 mtDNA 突变的同源人类黑色素瘤细胞杂交系。尽管氧化磷酸化功能失调,但具有同质水平致病性 mtDNA 的杂交种仍能可靠地形成肿瘤。然而,这些 mtDNA 变体破坏了原发性肿瘤的自发转移,并降低了循环肿瘤细胞的丰度。肿瘤细胞的迁移和侵袭也减少了,这表明在mtDNA功能失调的情况下,进入血液循环是转移的一个瓶颈。致病性 mtDNA 在静脉注射后不会抑制器官定植。在异质细胞杂交肿瘤中,单细胞分析显示黑色素瘤生长过程中对致病mtDNA的选择。总之,这些研究结果通过实验证明,功能性mtDNA在黑色素瘤生长过程中受到青睐,并支持转移到血液中。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Pathogenic mitochondrial DNA mutations inhibit melanoma metastasis
Mitochondrial DNA (mtDNA) mutations are frequent in cancer, yet their precise role in cancer progression remains debated. To functionally evaluate the impact of mtDNA variants on tumor growth and metastasis, we developed an enhanced cytoplasmic hybrid (cybrid) generation protocol and established isogenic human melanoma cybrid lines with wild-type mtDNA or pathogenic mtDNA mutations with partial or complete loss of mitochondrial oxidative function. Cybrids with homoplasmic levels of pathogenic mtDNA reliably established tumors despite dysfunctional oxidative phosphorylation. However, these mtDNA variants disrupted spontaneous metastasis from primary tumors and reduced the abundance of circulating tumor cells. Migration and invasion of tumor cells were reduced, indicating that entry into circulation is a bottleneck for metastasis amid mtDNA dysfunction. Pathogenic mtDNA did not inhibit organ colonization following intravenous injection. In heteroplasmic cybrid tumors, single-cell analyses revealed selection against pathogenic mtDNA during melanoma growth. Collectively, these findings experimentally demonstrate that functional mtDNA is favored during melanoma growth and supports metastatic entry into the blood.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


